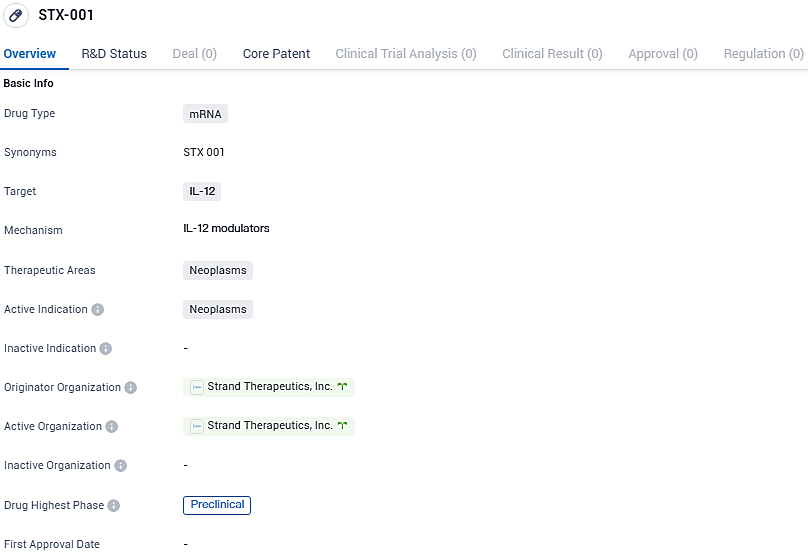
Strand Therapeutics Granted Authorization for Experimental RNA Drug STX-001, Aimed at Addressing Cancerous Growths
Strand Therapeutics, a pioneer in programmable mRNA technology for creating innovative treatments targeting cancer and various illnesses, recently revealed that the FDA has approved its request to commence a Phase 1 clinical trial, marking the initial human study of STX-001. This advanced construct harnesses synthetic self-amplifying mRNA technology designed to continuously produce the IL-12 cytokine within the tumor milieu.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"STX-001 is on the cusp of being a trailblazer as the pioneering treatment utilizing programmable mRNA in the cancer treatment landscape," proclaimed Dr. Jake Becraft, Chief Executive Officer and founding partner of Strand Therapeutics. "Obtaining the go-ahead on our IND application to proceed with STX-001 patient trials is a pivotal stride for Strand. The drug embodies a novel method in battling solid malignancies, and our ambition to push forward our programmable mRNA platforms in various medical applications is emboldened.”
STX-001 is emerging as a revolutionary method that might enhance the effectiveness of existing therapies for solid malignancies. The distinctiveness of STX-001 is its self-amplifying mRNA technology, which not only results in a type of cancer cell death that stimulates an immune response but also aids in attracting and triggering T cells and NK cells within the tumor milieu.
"Despite remarkable progress in the realm of immunotherapy for solid tumor conditions, there’s an overarching challenge where a majority of patients ultimately encounter resistance and experience an advancement of the disease whilst on treatment," Dr. Tasuku Kitada, a pioneer at Strand Therapeutics acting as President and chief of R&D stated.
Kitada elaborated, "Our pioneering work over recent years in programmable mRNA therapies has been geared towards delivering precise and targeted immune activation directly to the site of the tumor. The green light for STX-001 is a key evolution for our immune-focused cancer treatments, and we are anticipating the initiation of our very first trials with this candidate in the early stages of this year.”
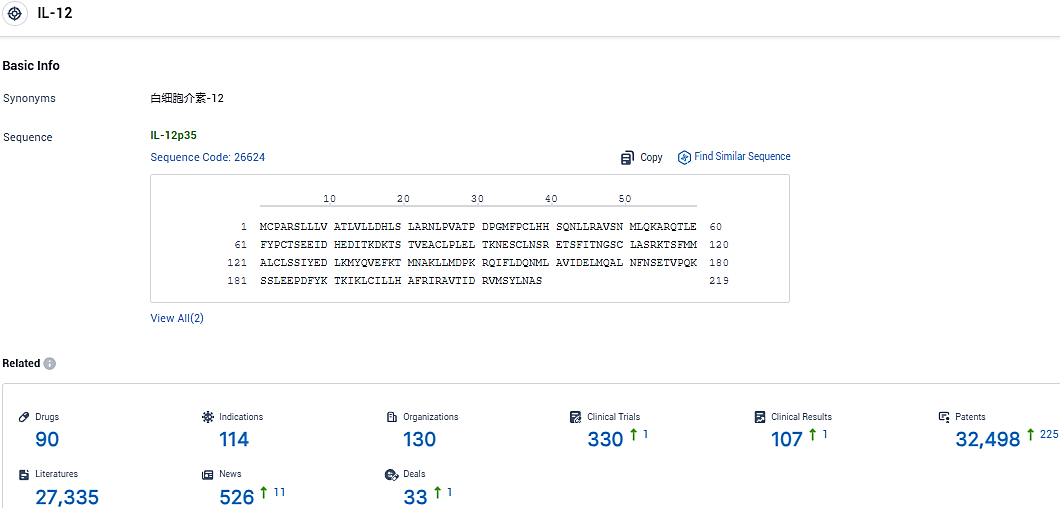
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 26, 2024, there are 90 investigational drugs for the IL-12 target, including 114 indications, 130 R&D institutions involved, with related clinical trials reaching 330, and as many as 32498 patents.
STX-001 is a promising drug candidate in the field of biomedicine, specifically targeting IL-12 in neoplasms. However, since it is still in the preclinical phase, further research and testing are required to determine its safety and efficacy in humans. As STX-001 progresses through the clinical phases, it will be important to closely monitor its development and evaluate its potential impact on neoplasms and patient outcomes.