Sanofi Plans to Acquire Inhibrx Inc for Rare Disease Candidate
Sanofi, a global healthcare leader, has established a firm acquisition deal to integrate Inhibrx, Inc., an exchange-listed company specializing in the research and advancement of a diverse range of innovative biologic treatments. The arrangement stipulates that Sanofi will take over Inhibrx after it separates its assets not related to INBRX-101 into a fresh entity, dubbed New Inhibrx.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
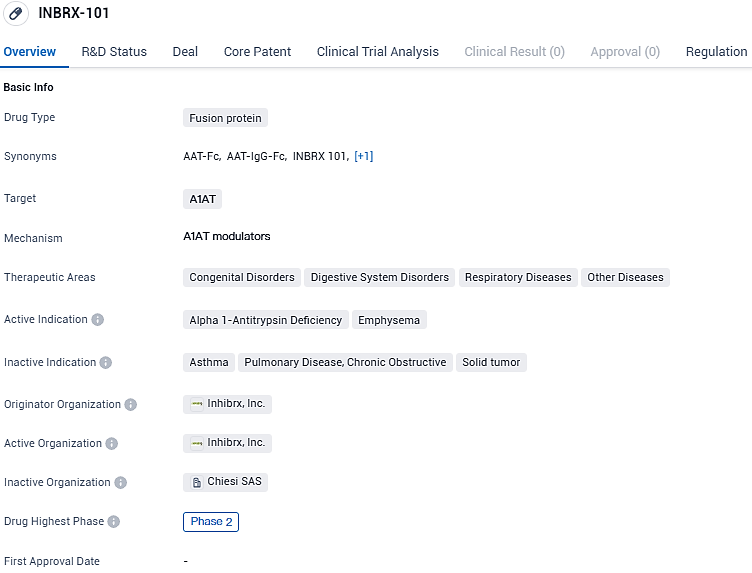
INBRX-101 is an engineered human protein envisioned to grant patients with Alpha-1 Antitrypsin Deficiency (AATD) the ability to maintain normalized serum AAT concentrations, potentially requiring less frequent administration. AATD is a genetic condition hallmarked by deficient AAT protein levels, primarily causing progressive lung damage. INBRX-101 could play a role in diminishing inflammation and slowing down the decline in pulmonary function in individuals with AATD.
"Integrating INBRX-101 into our catalog of unique therapies for rare diseases underscores our dedication to novel, top-tier product candidates. Leveraging our niche expertise in rare illnesses and expanding influence in immune-related respiratory disorders, INBRX-101 is anticipated to enhance our research and development initiatives in prioritized areas, aiming to fulfill the unmet needs of patients and communities grappling with AATD," commented Houman Ashrafian, Head of Research and Development at Sanofi.
Having achieved encouraging safety and pharmacokinetic findings in a completed Phase 1 study, INBRX-101 is now progressing into a Phase 2 clinical trial to ascertain its efficacy as a potential therapeutic agent for AATD. Should it prove successful, INBRX-101 stands to significantly elevate the therapeutic approaches and life quality for those living with AATD.
In the aftermath, New Inhibrx will maintain ownership of assets outside of INBRX-101, most noteworthy being its immuno-oncology programs (INBRX-109, INBRX-106, INBRX-105), as well as other Inhibrx properties not pertinent to INBRX-101 along with its personnel. Mark P. Lappe, the founder and current Chief Executive Officer of Inhibrx, is set to preside as the Chairman and CEO of the newly formed New Inhibrx, operating under the established Inhibrx brand.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
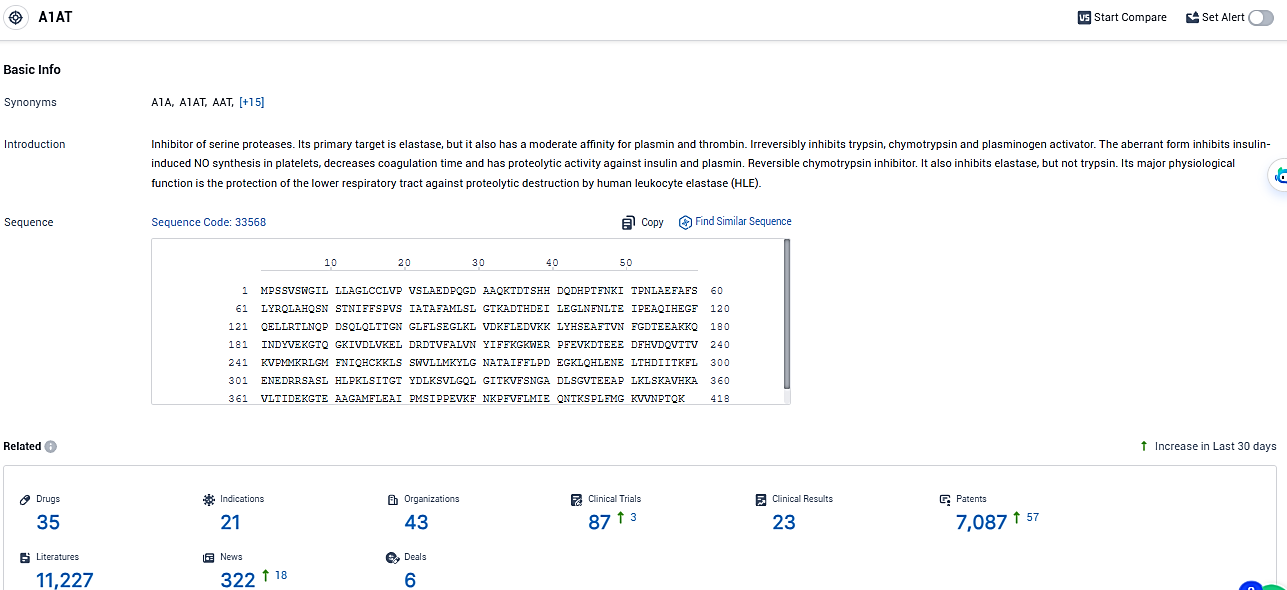 According to the data provided by the Synapse Database, As of January 26, 2024, there are 35 investigational drugs for the A1AT target, including 21 indications, 43 R&D institutions involved, with related clinical trials reaching 87, and as many as 7087 patents.
According to the data provided by the Synapse Database, As of January 26, 2024, there are 35 investigational drugs for the A1AT target, including 21 indications, 43 R&D institutions involved, with related clinical trials reaching 87, and as many as 7087 patents.
INBRX-101 targets A1AT and is primarily focused on treating Alpha 1-Antitrypsin Deficiency and Emphysema. It has reached Phase 2 of clinical trials and has received regulatory designations of Fast Track and Orphan Drug. Further research and development are required to determine the drug's efficacy and safety profile.