SIFI Gains European Commission Nod for AKANTIOR®
SIFI, a premier global ophthalmic enterprise, revealed that the European Commission has granted approval for AKANTIOR® (polihexanide) for managing acanthamoeba keratitis (AK) in both adults and children aged 12 and over, thereby affirming the product's orphan designation. AKANTIOR stands as the first and sole authorized treatment for individuals affected by AK in Europe.
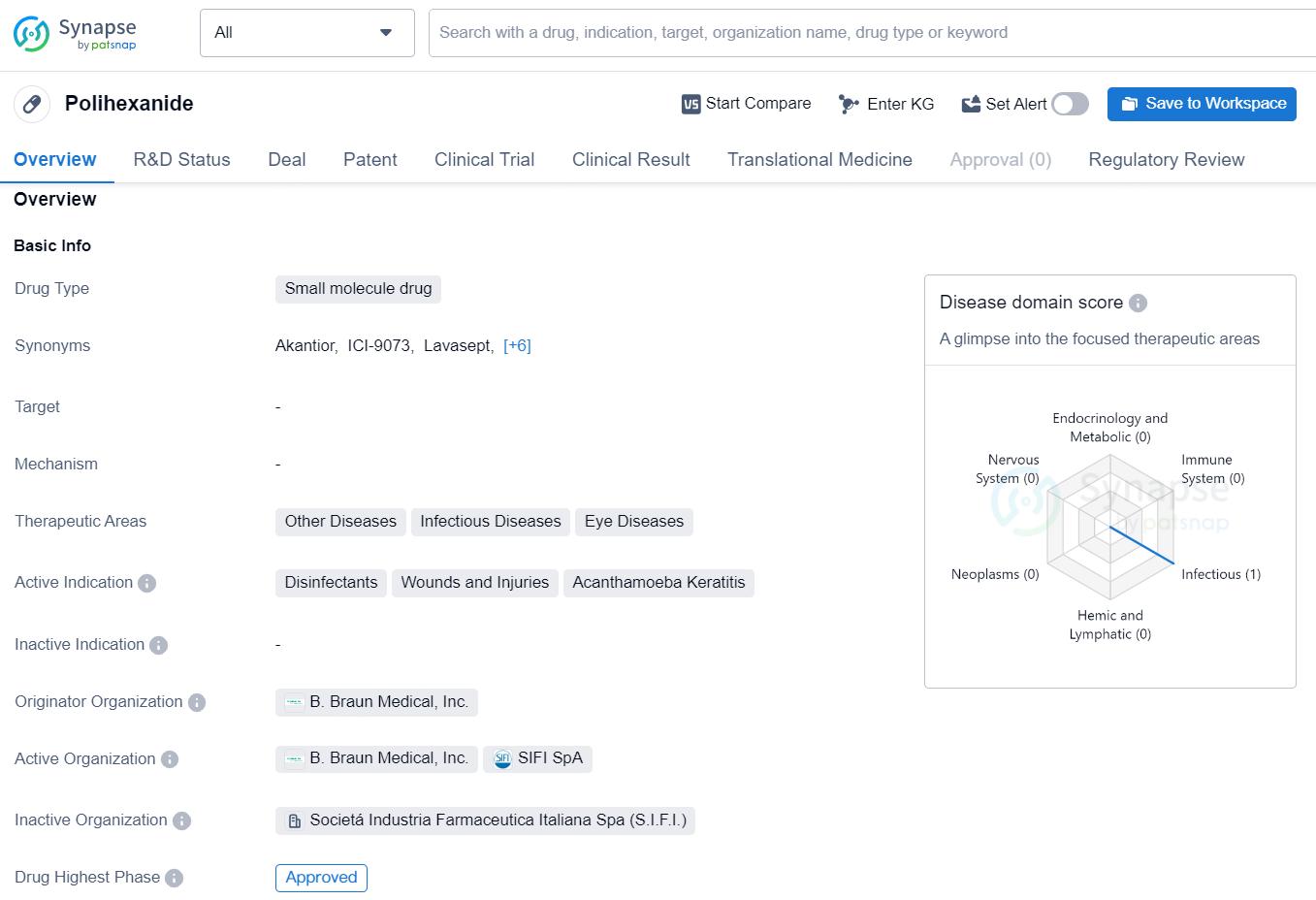
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
AK is a highly uncommon, severe, and progressive infection of the cornea that can threaten vision, characterized by intense pain and light sensitivity. This condition is caused by a free-living amoeba known as acanthamoeba, with contact lens users being primarily affected.
“Today’s approval is a significant milestone toward establishing a new global treatment standard for AK patients, reflecting over 15 years of dedicated research and development,” stated Fabrizio Chines, Chairman and CEO of SIFI. “For the first time, Europeans with AK have access to an approved therapy, heralding a new chapter in managing this potentially blinding disease. AKANTIOR stands as the first and only approved medication for AK, and our team remains dedicated to exploring polihexanide applications in other corneal infections, including fungal keratitis, where it has received orphan drug status from both the EMA and FDA.”
“Used as a monotherapy according to the trial’s treatment protocol, AKANTIOR achieves medical cure rates exceeding 86%, establishing it as the standard care for this serious and debilitating condition,” explained Professor John Dart from Moorfields Eye Hospital and UCL Institute of Ophthalmology, Principal Investigator of the Phase III ODAK trial. “Following 15 years of rigorous research and development by SIFI culminating in the pivotal Phase III clinical trial, the European Medicines Agency and European Commission have approved polihexanide 0.08% as the first licensed treatment for acanthamoeba keratitis. This development represents a substantial improvement over current treatment options and has the potential to preserve sight and prevent blindness. As the only approved treatment, it is recommended for use with the specific protocol from the Phase 3 trial, eliminating the need for the variable individualized treatments currently in practice, and offering an effective, standardized approach that any clinician can follow, proven effective not only in trials but also in current compassionate use cases.”
“With the approval of AKANTIOR, AK patients (referred to as AK Warriors) are now closer to accessing top-quality treatment within the EU. This milestone is a significant step toward equitable healthcare for all AK Warriors, providing hope for a better future,” remarked Juliette Vila Sinclair Spence, a rare disease patient advocate and the Chairwoman and Founder of the AK Eye Foundation—the first global foundation exclusively dedicated to this condition.
In Europe, SIFI plans to commence its first commercial launch in Germany in the last quarter of this year, followed by entry into additional markets contingent on local regulatory approvals, healthcare technology assessments, and reimbursement processes. This will include France, Italy, Romania, Spain, the United Kingdom, and Turkey, covering a total population of 430 million, with further markets to be addressed by its commercial partner, Avanzanite, including other European countries with an additional population of 180 million. According to scientific publications, the incidence of acanthamoeba keratitis ranges from 1 to 4 patients per million inhabitants.
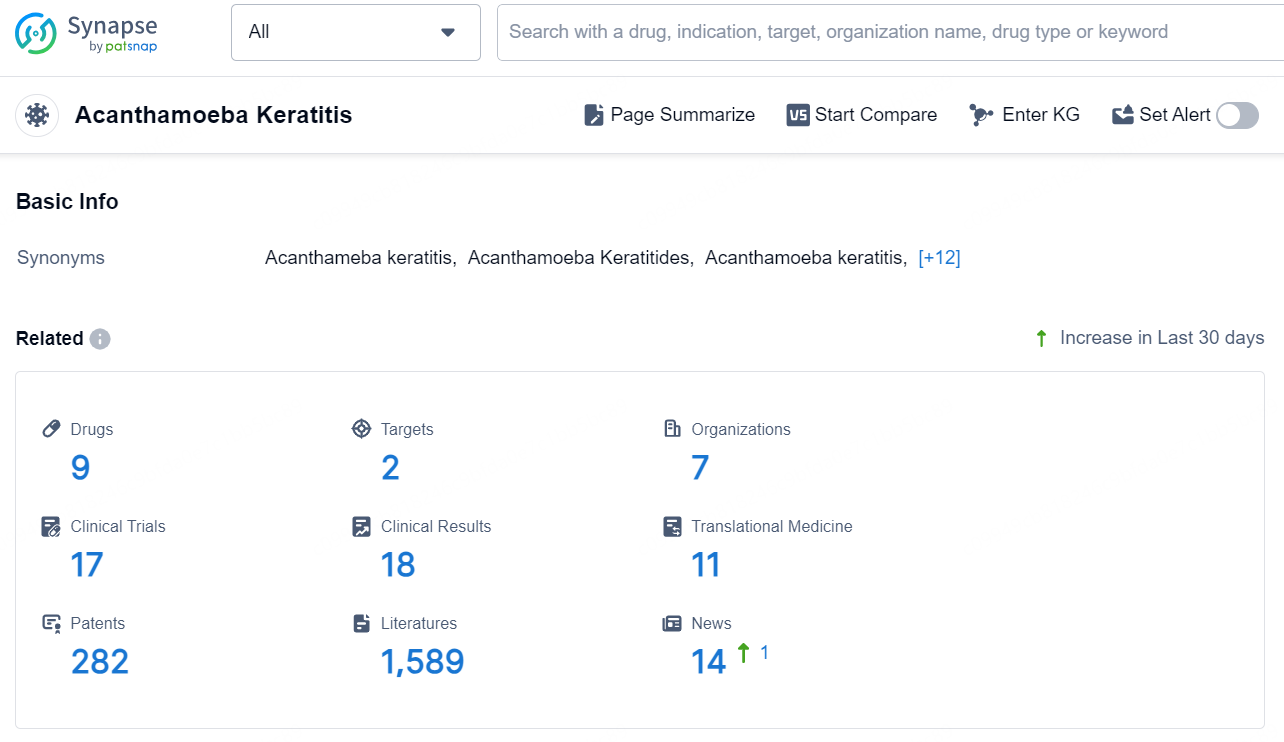
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 27, 2024, there are 9 investigational drugs for the Acanthamoeba Keratitis, including 2 targets, 7 R&D institutions involved, with related clinical trials reaching 17, and as many as 282 patents.
Polihexanide is a small molecule drug used in the treatment of various therapeutic areas, including other diseases, infectious diseases, and eye diseases. Its active indications include disinfectants, wounds and injuries, and Acanthamoeba Keratitis. The drug is developed by B. Braun Medical, Inc., and it has reached the highest phase of global approval.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!