Skyhawk Therapeutics Announces Successful Phase 1 Results for SKY-0515 in Huntington's Disease
Skyhawk Therapeutics, Inc., a biotechnology firm in the clinical stage focusing on innovative small molecule treatments to target key RNA molecules, has shared encouraging outcomes from Parts A and B of its Phase 1 clinical trial for SKY-0515, aimed at treating Huntington's disease. The trial results indicated that SKY-0515 achieved an average reduction of 72% in HTT mRNA at a daily oral dose of 9mg and showed good tolerance across all tested dosages.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
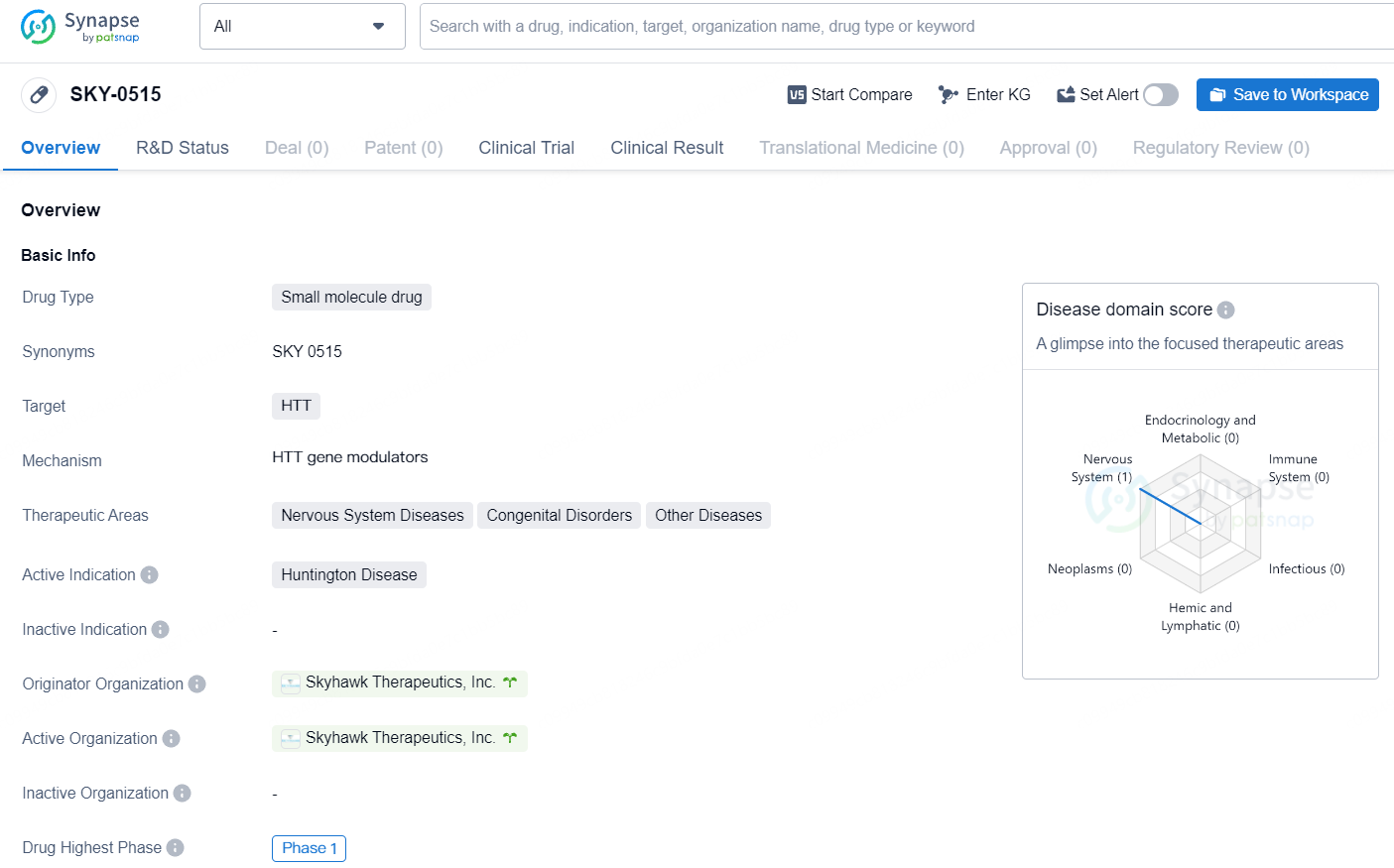 SKY-0515 is Skyhawk's investigational small molecule RNA splicing modifier, developed using the company's innovative RNA-splicing platform. The purpose of SKY-0515 is to lower the levels of both HTT and PMS1 proteins, the latter being another significant factor in somatic CAG repeat expansion and Huntington’s Disease (HD) pathology.
SKY-0515 is Skyhawk's investigational small molecule RNA splicing modifier, developed using the company's innovative RNA-splicing platform. The purpose of SKY-0515 is to lower the levels of both HTT and PMS1 proteins, the latter being another significant factor in somatic CAG repeat expansion and Huntington’s Disease (HD) pathology.
"Huntingtin reduction and somatic expansion have been two of the most active research areas in HD over the past ten years. Lowering both HTT and PMS1 may provide greater therapeutic benefits than targeting HTT alone," stated Ed Wild, Professor of Neurology at University College London. "Huntington’s disease is a rare, inherited neurodegenerative disorder that affects over 40,000 individuals in the United States. There are currently no treatments available that can halt or slow the progression of the disease. The HTT reduction seen with SKY-0515 has the broadest dynamic range I have observed in any therapeutic approach, offering significant hope that it may someday benefit those afflicted by the disease."
"We believe that with the notable decrease in HTT mRNA and the predicted suppression of PMS1 protein, SKY-0515, if approved, could substantially improve the lives of Huntington’s patients," commented Douglas V. Faller, M.D., Ph.D., Chief Medical Officer at Skyhawk Therapeutics. "The Safety Review Committee has found that SKY-0515 is generally well tolerated at all tested doses, with systemic exposure increasing proportionally with dose. In light of these favorable safety findings, the study is now proceeding to the patient phase. Recruitment is underway, and top-line data from this segment of the trial are projected to be released in Q2 2025."
"Since the launch of this Phase 1 clinical trial in late 2023, we are pleased with the rapid progression of the study and are excited to present such promising results for SKY-0515," said Clint Musil, Chief Executive Officer of Skyhawk Therapeutics. "These initial data mark a significant milestone for SKY-0515 and highlight the vast potential of the Skyhawk platform to address conditions that currently lack approved disease-modifying treatments."
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
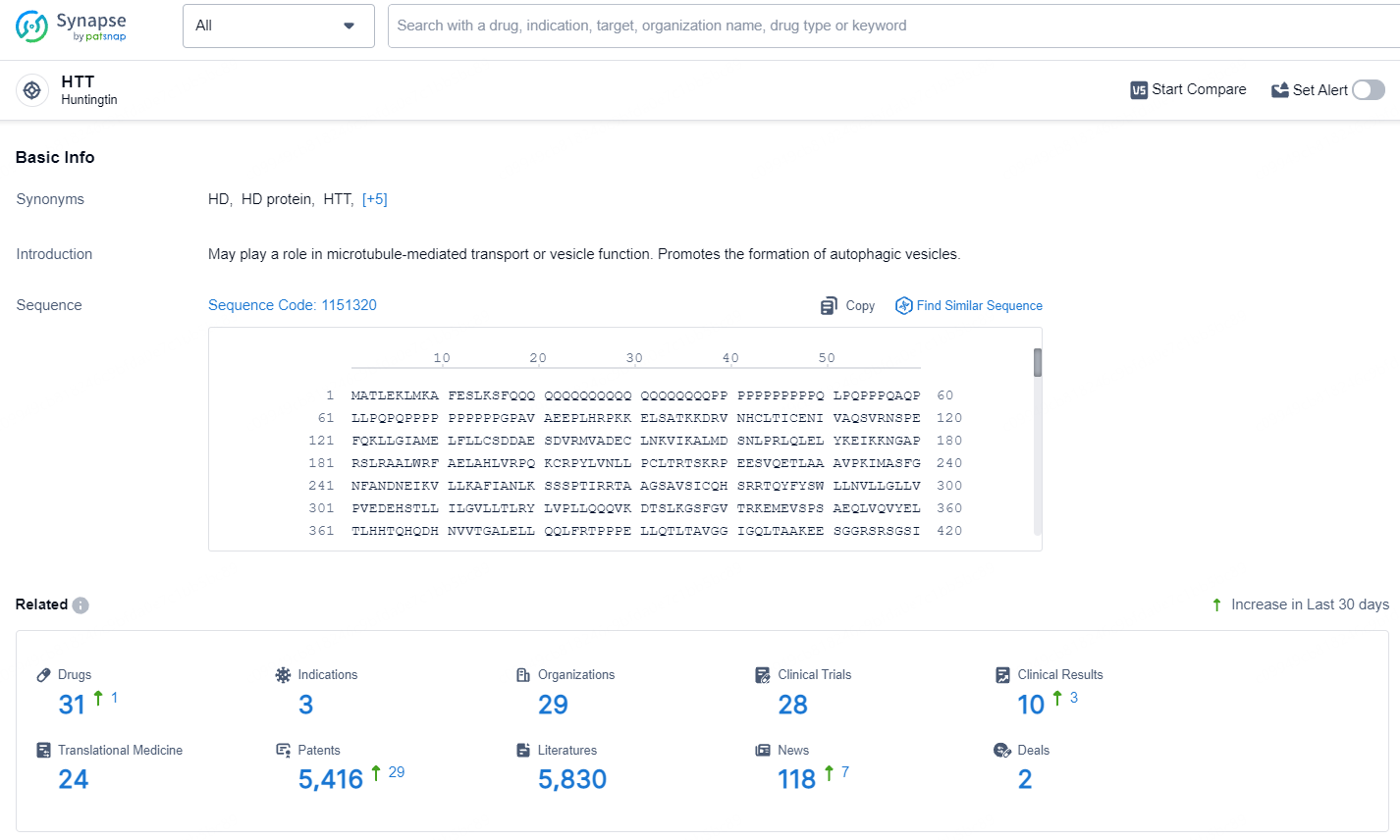 According to the data provided by the Synapse Database, As of July 16, 2024, there are 31 investigational drugs for the HTT target, including 3 indications, 29 R&D institutions involved, with related clinical trials reaching 28, and as many as 5416 patents.
According to the data provided by the Synapse Database, As of July 16, 2024, there are 31 investigational drugs for the HTT target, including 3 indications, 29 R&D institutions involved, with related clinical trials reaching 28, and as many as 5416 patents.
Further studies and clinical trials will be essential to determine the full potential and efficacy of SKY-0515 in treating the targeted diseases. The small molecule nature of the drug may offer advantages in terms of targeted delivery and potential for further development in addressing unmet medical needs in the specified therapeutic areas.