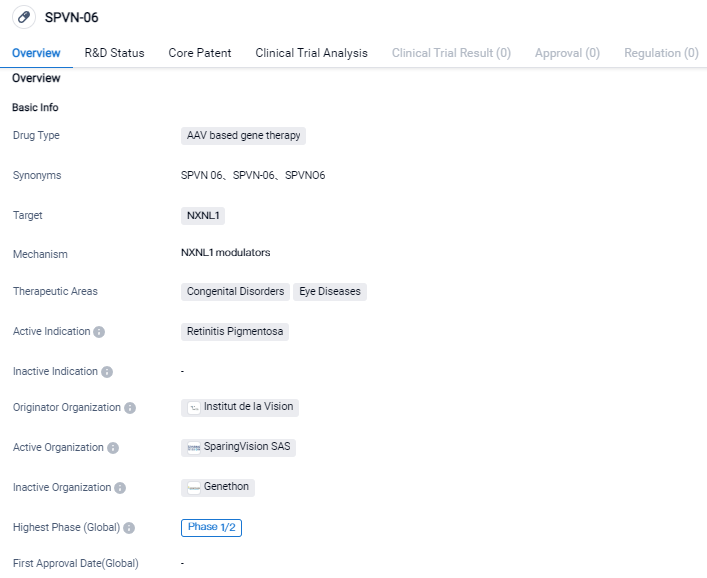
SparingVision announces encouraging safety Data in its phase I/II PRODYGY Gene Therapy Trial of SPVN06
SparingVision, a genomic medicine corporation in the clinical-stage phase that's working on creating treatments to preserve sight in ocular disease cases, has shared encouraging early safety data from the Phase I/II clinical trial of its primary gene-agnostic gene therapy, SPVN06, designed to treat retinitis pigmentosa. The innovative gene therapy strategy of SPVN06 targets preventing or decelerating disease evolution in patients sufering from rod-cone dystrophy, irrespective of their genetic makeup. Initially, SparingVision is concentrating on mid-stage RP, a significant hereditary culprit of worldwide blindness.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Safety-related data from the initial three patients receiving a low dosage found SPVN06 to be well received with a positive safety record. These patients each received injections at the 15-20 National Hospital situated in Paris, France. Upon reviewing the data, the Data Safety Monitoring Board has deemed it safe to commence with the second group on a medium dosage.
SparingVision's President and CEO, Stéphane Boissel said: "Our focus is to safeguard vision using innovative genomic techniques, and it's our strong belief that SPVN06 has the potential to considerably alter patients' lives and provide hope to those suffering from retinitis pigmentosa. This trial marks the start, we have an extensive range of vision-preserving treatments that utilize advanced genomic techniques set to revolutionize the existing treatment of retinal diseases. We are eager to update on the progress of SPVN06 and our other initiatives as we swiftly advance our portfolio through the development phase."
Avril Daly, Retina International’s CEO, a group advocating for patients, commented: “The encouraging initial safety results with SPVN06 indicates great progress for reitinitis pigmentosa patients. As a community, over the years, we've emphasized the need for a cure accommodating the vast genetic diversities causing RP. SparingVision's strategy with SPVN06 brings hope to those of us who have been affected by this debilitating blindness-causing disease. We are anxiously anticipating the outcomes of this Phase I/II trial and the therapy's potential to halt or slow down the progression of RP."
The primary outcome of the PRODYGY Phase I/II trial is anticipated to be attained in 2025, and early effectiveness results are predicted to be available within the same year.
PRODYGY is multi-centric Phase I/II trial designed to evaluate the safety, patient tolerance also the initial effectiveness and the quality of life post a single subretinal injection of SPVN06 in the eye with the worst vision of adult patients with RCD due to a RHO, PDE6A, or PDE6B gene mutation. The study will enlist around 33 patients in two stages.
• Stage 1: An open-label, dose-increasing phase comprising three groups of three patients each with severe advanced RCD, with the aim to identify the two best-tolerated dosages for the second step.
• Stage 2: A double-masked, controlled, randomized expansion phase comprising 24 patients with advanced intermittent RP, grouped into three cohorts: six patients without treatment and 18 patients administered with one of the two recommended dosages determined in Stage One.
SPVN06 is a proprietary, mutation-blind, AAV gene therapeutic course encompassing one neurotrophic factor (Rod-derived Cone Viability Factor), and one oxidative stress reducing enzyme (Rod-derived Cone Viability Factor Long form). Working together, RdCVF and RdCVFL aim to halt or slow the degeneration of cone photoreceptors, a condition that inevitably leads to blindness in patients with rod-cone dystrophies. Principal disease targets for SparingVision are retinitis pigmentosa, a prevalent inherited retinal ailment affecting approximately two million patients globally, and dry Age-related Macular Degeneration. No treatment is currently approved to cater to RP patients irrespective of their genetic diversities. This strategy could be potentially applied to a number of diseases, where rod loss is an early warning sign of the illness. SPVN06 is a product of decades of top-tier ophthalmology research by SparingVision creators José-Alain Sahel and Thierry Léveillard at the Paris Vision Institute.
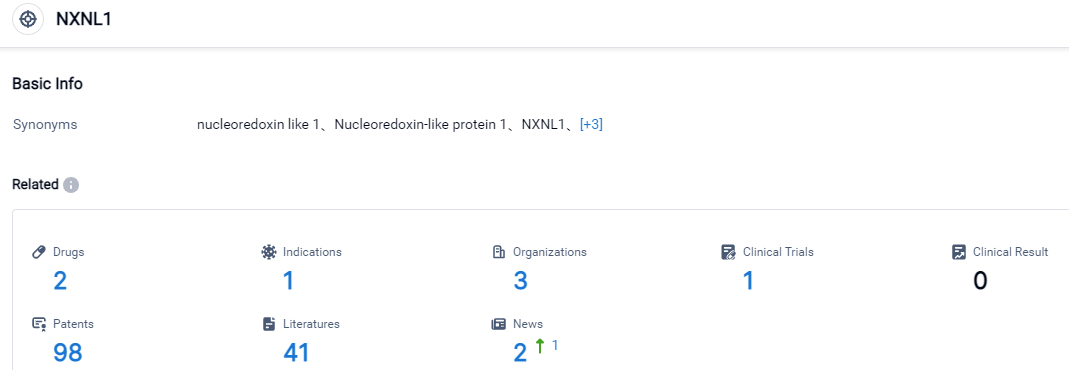
Click on the image below for direct access to the latest R&D progress on NXNL1 target drugs, indications, research institutions, clinical trials, and more. As of August 31, 2023, there are 2 investigational drugs for the NXNL1 target, including 1 applicable indications, 3 R&D institutions involved, with related clinical trials reaching 1, and as many as 98 patents. SparingVision has developed a robust collection of modern gene therapy and genome editing treatments for inherited retinal diseases (IRDs). Its two main products, SPVN06 and SPVN20, aim to surpass single gene correction therapies by offering new gene-independent treatments for Retinitis Pigmentosa (RP), a cluster of IRDs chiefly causing blindness worldwide. Moreover, the company is strategically teaming up with Intellia Therapeutics (NASDAQ: NTLA) for creating unique genome editing-based remedies for ocular diseases using CRISPR-Cas9 technology.