Methimazole: Detailed Review on its Transformative R&D Success, Mechanism of Action, and Drug Targets
Methimazole's R&D Progress
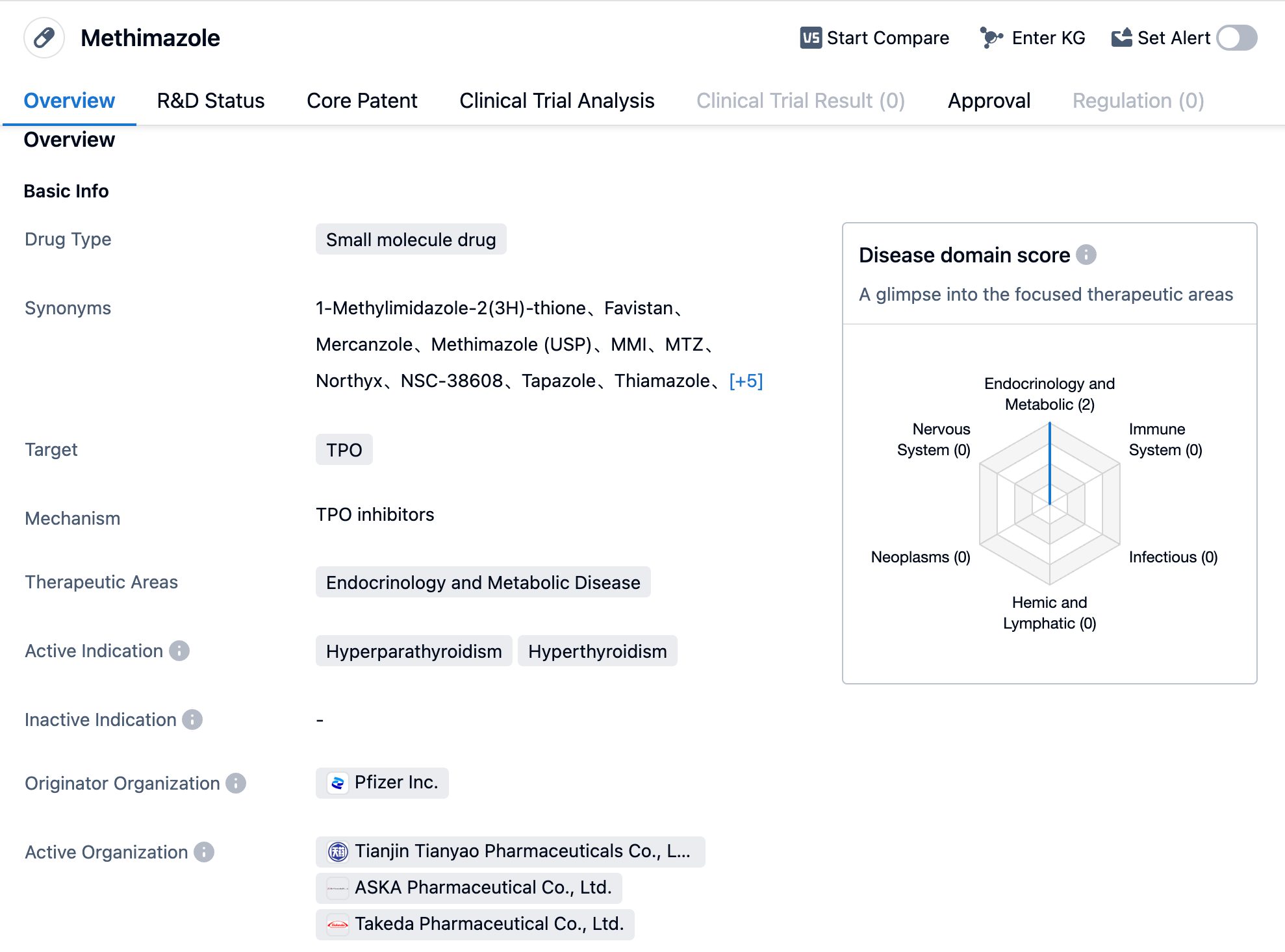
Methimazole is a small-molecule drug that falls under the therapeutic areas of endocrinology and metabolic disease. It primarily targets the enzyme thyroid peroxidase (TPO). The drug has been approved for the treatment of hyperparathyroidism and hyperthyroidism.
Methimazole was first approved in the United States in June 1950, making it one of the oldest drugs on the market for the treatment of these conditions. The drug is manufactured by Pfizer Inc., a renowned pharmaceutical company.
As a small molecule drug, Methimazole is designed to interact with specific molecular targets in the body, in this case, TPO. By targeting TPO, Methimazole helps to regulate the production of thyroid hormones, which are responsible for the proper functioning of the thyroid gland. This mechanism of action makes Methimazole an effective treatment option for hyperparathyroidism and hyperthyroidism.
Hyperparathyroidism is a condition characterized by the overactivity of the parathyroid glands, leading to excessive production of parathyroid hormone (PTH). Methimazole helps to reduce the levels of PTH, thereby restoring the balance of calcium and phosphorus in the body.
Hyperthyroidism, on the other hand, is a condition where the thyroid gland produces an excessive amount of thyroid hormones. Methimazole works by inhibiting the production of these hormones, thereby alleviating the symptoms associated with hyperthyroidism.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Methimazole: TPO inhibitor
A TPO inhibitor refers to a type of medication or compound that inhibits the activity of thrombopoietin (TPO), a hormone involved in the production of platelets in the body. From a biomedical perspective, TPO inhibitors are primarily used in the treatment of certain blood disorders, such as immune thrombocytopenia (ITP) and aplastic anemia, where there is a low platelet count. By blocking the action of TPO, these inhibitors can help increase platelet production and improve blood clotting. This can be beneficial in managing bleeding episodes and reducing the risk of excessive bleeding. TPO inhibitors may be administered orally or through injection, and their usage is typically monitored by healthcare professionals to ensure optimal dosing and minimize potential side effects.
Drug Target R&D Development for Methimazole
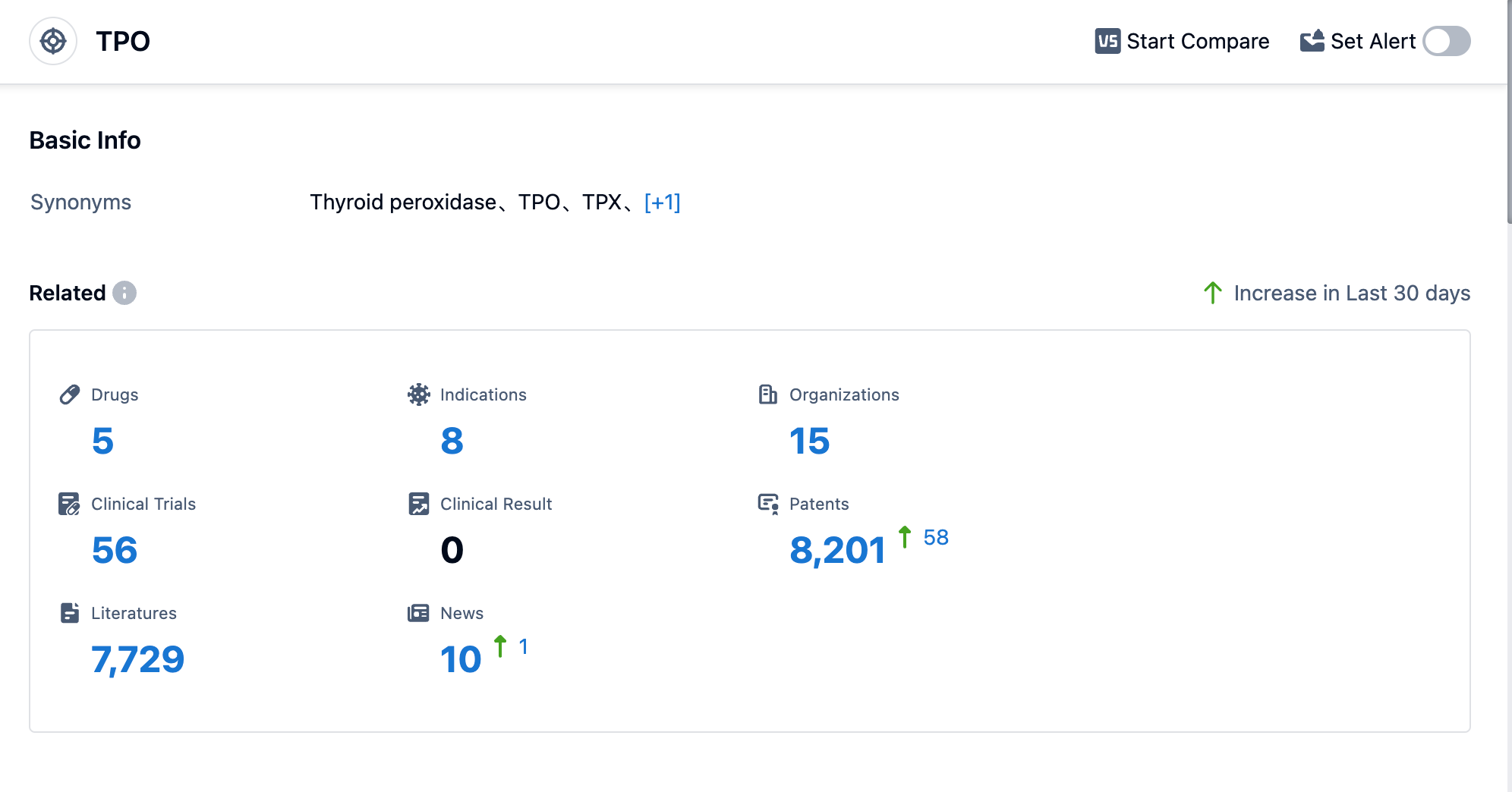
According to Patsnap Synapse, as of 31 Aug 2023, there are a total of 5 TPO drugs worldwide, from 15 organizations, covering 8 indications, and conducting 56 clinical trials.
Based on the analysis of the provided data, the current competitive landscape for target TPO is characterized by the growth of companies such as Takeda Pharmaceutical Co., Ltd., Shanghai Liuliguang Pharmaceutical Development Co., Ltd., and ASKA Pharmaceutical Holdings Co., Ltd. These companies have reached the highest stage of development and have made significant progress in their R&D efforts.
The approved drugs under the target TPO primarily target indications such as hyperthyroidism, hyperparathyroidism, Graves Disease, goiter, glioblastoma, stroke, myocardial infarction, and thrombocythemia, essential. This indicates the potential effectiveness of targeting TPO in treating various thyroid-related disorders and other conditions.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Methimazole is a small-molecule drug developed by Pfizer Inc. It targets TPO and is approved for the treatment of hyperparathyroidism and hyperthyroidism. With its first approval dating back to 1950 in the United States, Methimazole has a long-standing history in the pharmaceutical industry. Its approval in global markets further solidifies its position as an effective treatment option for these endocrine disorders.