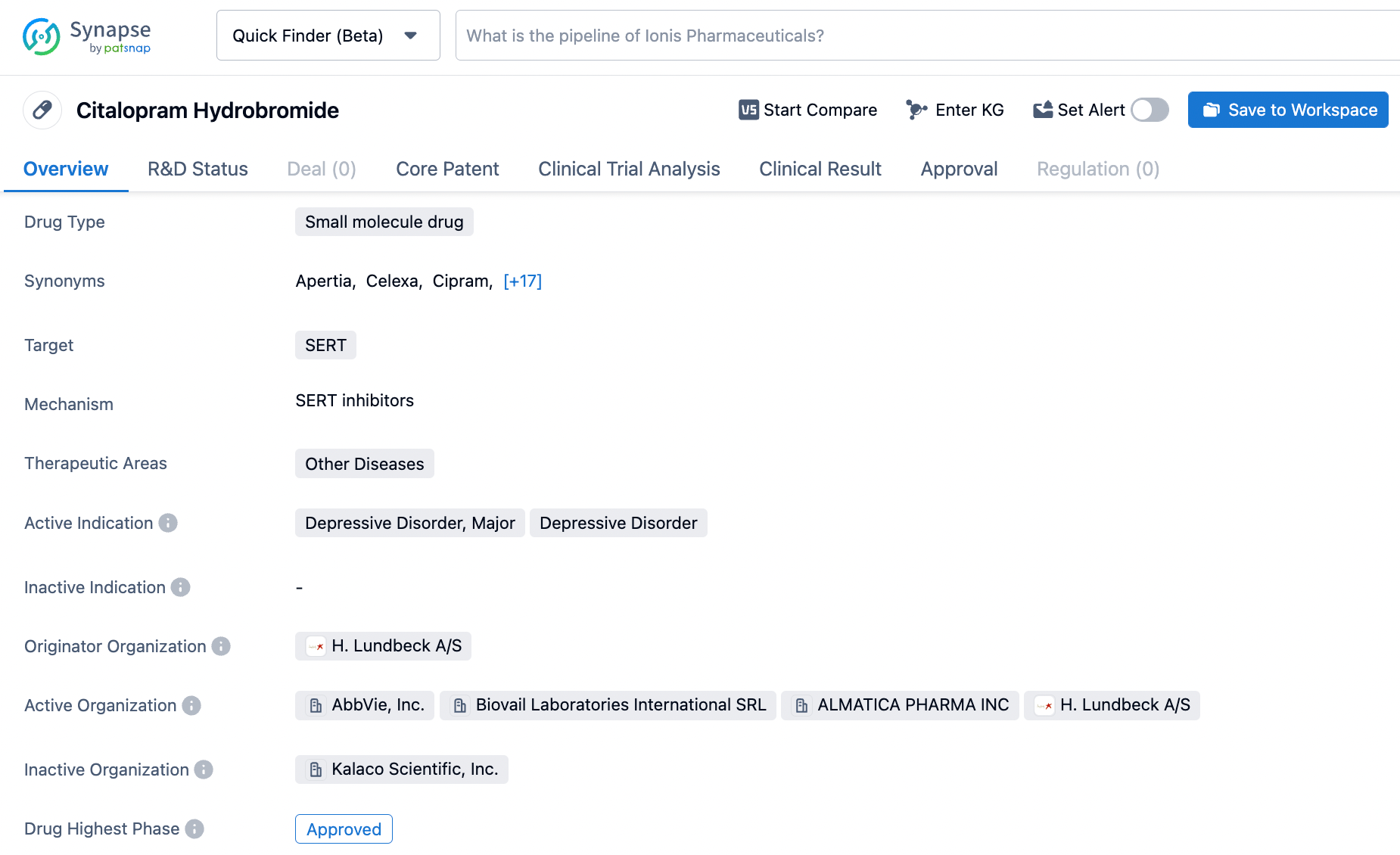
Strategically Search Citalopram on Synapse: A How-to Guide
Citalopram was approved for medical use in the United States in 1998. It is on the World Health Organization's List of Essential Medicines. Citalopram, sold under the brand name Celexa among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used to treat major depressive disorder, obsessive compulsive disorder, panic disorder, and social phobia. The antidepressant effects may take one to four weeks to occur. It is taken by mouth. Common side effects include nausea, trouble sleeping, sexual problems, shakiness, feeling tired, and sweating. Serious side effects include an increased risk of suicide in those under the age of 25, serotonin syndrome, glaucoma, and QT prolongation. Antidepressant discontinuation syndrome or persistent Post-SSRI sexual dysfunction may occur when stopped. Click on the image below to begin the exploration journey of Citalopram through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!