Pharmaceutical Insights: Sodium zirconium cyclosilicate's R&D Progress
Sodium zirconium cyclosilicate's R&D Progress
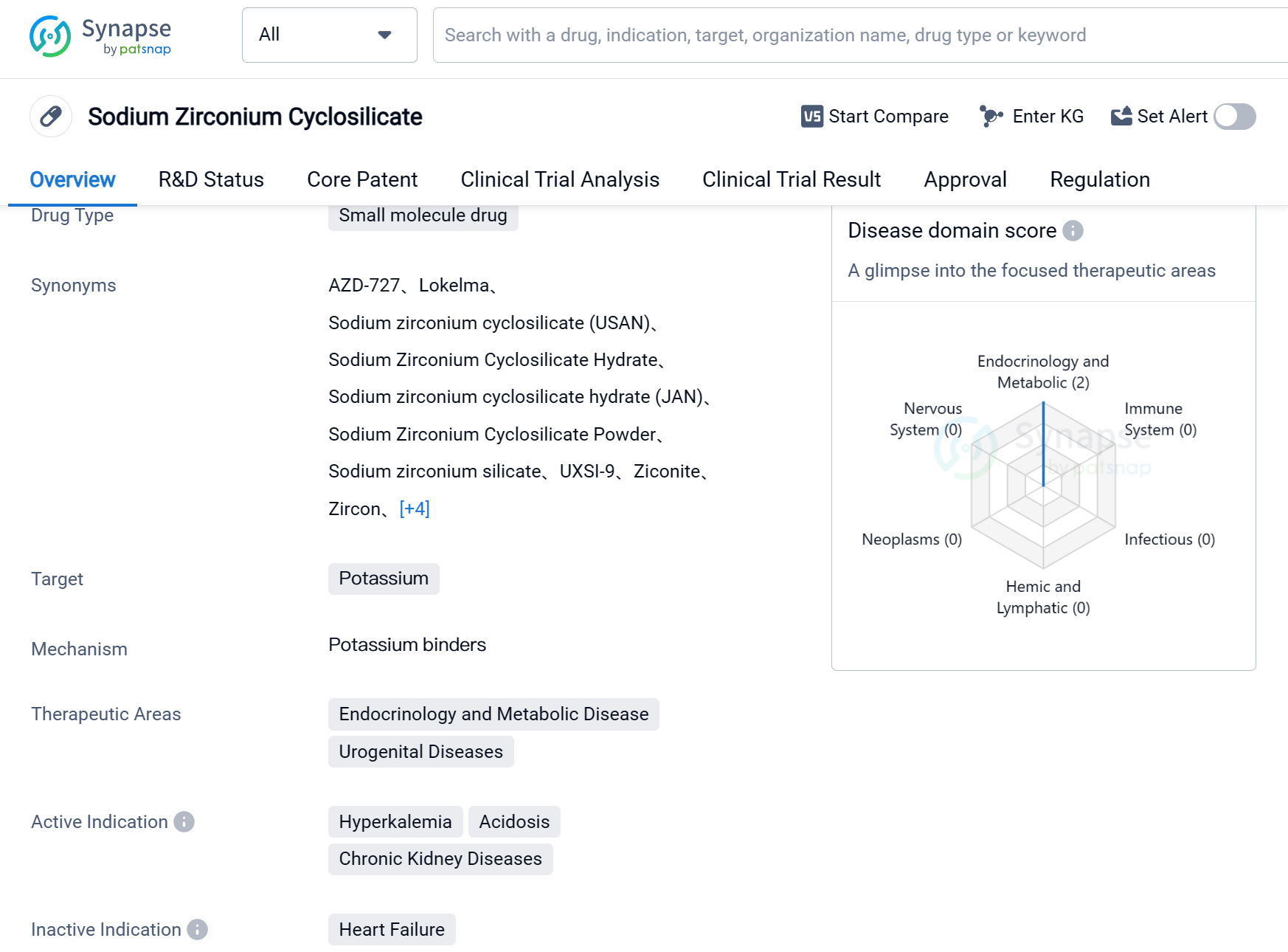
Sodium Zirconium Cyclosilicate is a small molecule drug that targets potassium and is used in the treatment of various conditions related to endocrinology, metabolic disease, urogenital diseases, and chronic kidney diseases. It is primarily indicated for the management of hyperkalemia, acidosis, and chronic kidney diseases.
The drug was developed by AstraZeneca Pharmaceutical Co., Ltd., a renowned pharmaceutical organization. It has received approvals globally. The drug obtained its first approval in the European Union in March 2018.
Sodium Zirconium Cyclosilicate falls under the category of overseas new drugs urgently needed in clinical settings and has been granted fast track status. This regulatory designation highlights the importance and urgency of the drug in addressing unmet medical needs.
Hyperkalemia, a condition characterized by high levels of potassium in the blood, can lead to serious cardiac complications. Sodium Zirconium Cyclosilicate offers a promising solution for managing this condition effectively. Additionally, the drug's therapeutic potential extends to the treatment of acidosis, a condition characterized by excessive acidity in the body, and chronic kidney diseases.
As a small molecule drug, Sodium Zirconium Cyclosilicate is designed to target specific molecular pathways involved in potassium regulation. By modulating these pathways, the drug helps restore potassium balance in the body, thereby alleviating the symptoms associated with hyperkalemia, acidosis, and chronic kidney diseases.
The approval of Sodium Zirconium Cyclosilicate in multiple regions reflects its potential to address significant medical needs globally. Its fast track designation further emphasizes the urgency and importance of this drug in clinical practice.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Sodium zirconium cyclosilicate: Potassium binder
Potassium binders are a type of medication used in the field of biomedicine to treat hyperkalemia, which is a condition characterized by high levels of potassium in the blood. These binders work by binding to potassium ions in the gastrointestinal tract, preventing their absorption into the bloodstream. By reducing the amount of potassium absorbed, potassium binders help lower the overall levels of potassium in the body.
Hyperkalemia can be caused by various factors, such as kidney dysfunction, certain medications, or underlying medical conditions. It can lead to serious complications, including cardiac arrhythmias and muscle weakness. Potassium binders play a crucial role in managing hyperkalemia and preventing these complications.
There are different types of potassium binders available, including sodium polystyrene sulfonate (SPS) and patiromer. SPS works by exchanging sodium ions for potassium ions in the intestines, while patiromer binds to potassium in exchange for calcium ions. These medications are typically taken orally and can be prescribed alongside other treatments to help regulate potassium levels in the body.
It is important to note that the use of potassium binders should be done under the guidance and supervision of a healthcare professional. The dosage and duration of treatment may vary depending on the individual's condition and medical history. Regular monitoring of potassium levels is also necessary to ensure the effectiveness and safety of the medication.
Drug Target R&D Trends for Sodium zirconium cyclosilicate
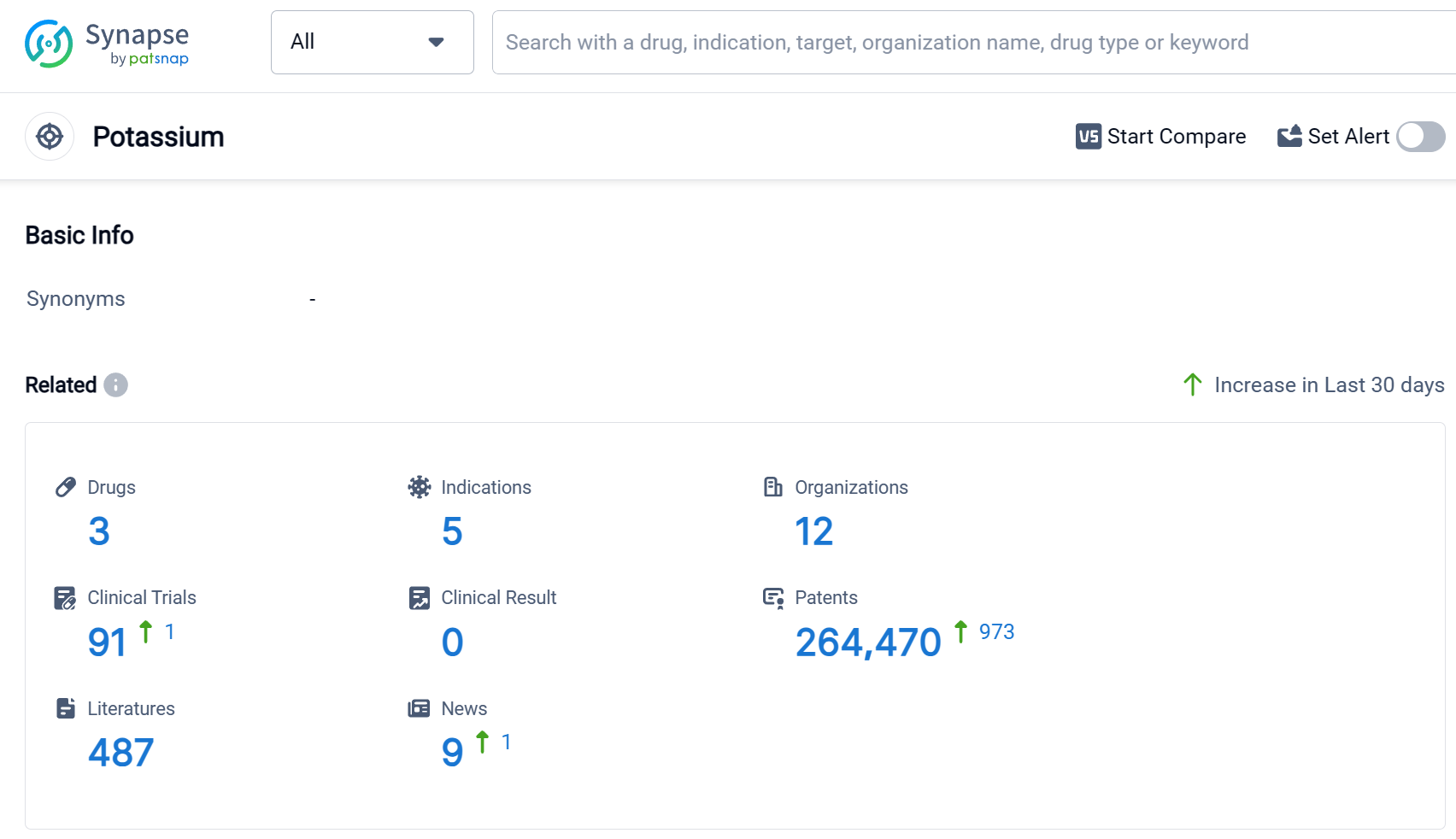
According to Patsnap Synapse, as of 3 Sep 2023, there are a total of 3 Potassium drugs worldwide, from 12 organizations, covering 5 indications, and conducting 91 clinical trials.
The analysis of the current competitive landscape of target Potassium reveals that CSL Ltd., AstraZeneca PLC, Vifor Fresenius Medical Care Renal Pharma Ltd., Otsuka Holdings Co., Ltd., Vifor Fresenius Medical Care Renal Pharma France S.A.S., and Zeria Pharmaceutical Co., Ltd. are the companies growing fastest under this target. These companies have drugs in advanced stages of development, including Approved and Phase 3 phases. The indications targeted by these drugs include Hyperkalemia, Chronic Kidney Diseases, Acidosis, Heart Failure, and Hypertension. Small molecule drugs are progressing rapidly under this target, with a focus on developing innovative treatments. The countries/locations developing fastest include the European Union, the United States, Canada, and China, among others. The global effort in the development of drugs targeting Potassium indicates a promising future for the advancement of treatments in this area.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Sodium Zirconium Cyclosilicate is a small molecule drug developed by AstraZeneca Pharmaceutical Co., Ltd. It targets potassium and is indicated for the treatment of hyperkalemia, acidosis, and chronic kidney diseases. The drug has received approvals in the European Union and China, and its fast track status highlights its significance in addressing unmet medical needs. Sodium Zirconium Cyclosilicate offers a promising solution for patients suffering from these conditions, providing a potential breakthrough in the field of biomedicine.