SUPERNOVA Phase III Trial: Sipavibart Effectively Prevents COVID-19 in Immunocompromised Patients
Encouraging top-line results from the SUPERNOVA Phase III trial for COVID-19 pre-exposure prophylaxis revealed that AstraZeneca's investigational long-acting antibody, sipavibart (previously known as AZD3152), significantly reduced the occurrences of symptomatic COVID-19 in comparison to the control group (either tixagevimab/cilgavimab or placebo) within an immunocompromised cohort.
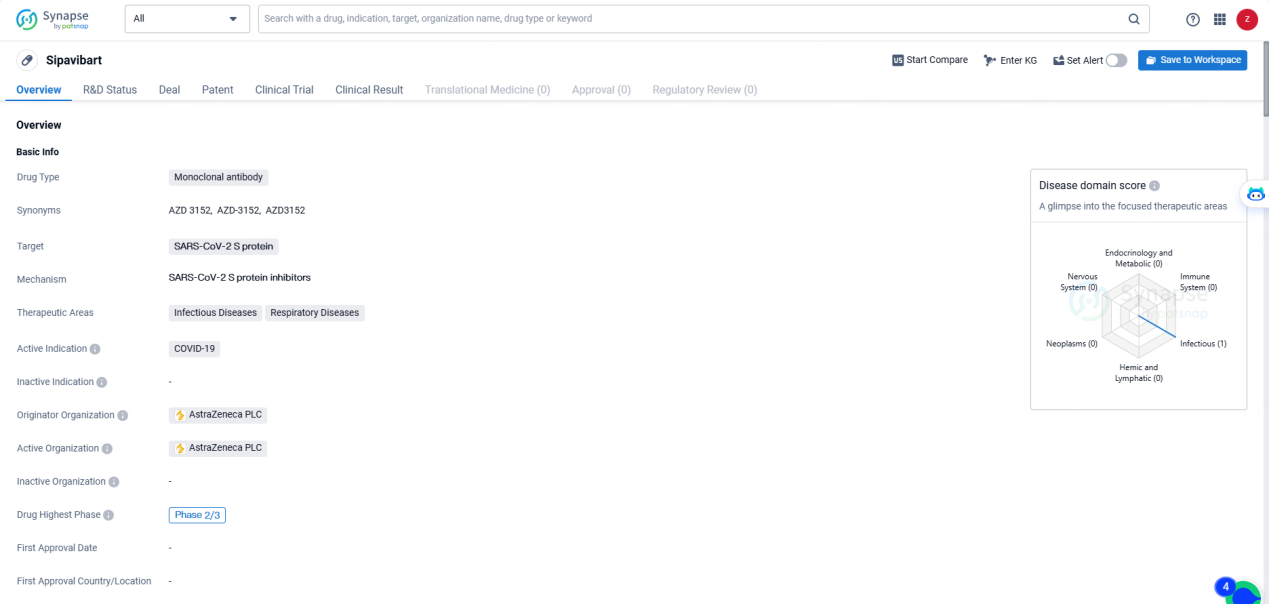
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The trial achieved success in both of its primary objectives: the first being the reduction in relative risk of symptomatic COVID-19 caused by any SARS-CoV-2 variant, and the second being the reduction in relative risk of infections by SARS-CoV-2 variants lacking the F456L mutation. The SUPERNOVA study illustrated the potential advantages of sipavibart in a landscape of evolving variants, with COVID-19 cases observed during the trial attributed to multiple different SARS-CoV-2 variants.
SUPERNOVA is an extensive Phase III international trial that provides unique efficacy data for immunocompromised patients, showcasing the potential benefits of a COVID-19 antibody against recent SARS-CoV-2 variants. Immunocompromised individuals include those with hematological cancers, organ transplant recipients, patients with end-stage renal disease on dialysis, individuals who have received B-cell depleting therapy in the past year, and those on immunosuppressive drugs.
Although immunocompromised patients make up around 4% of the population, they account for approximately 25% of COVID-19 hospitalizations, ICU admissions, and deaths, even after receiving multiple doses of COVID-19 vaccines.
Ghady Haidar, M.D., a UPMC transplant infectious diseases physician and primary investigator for the SUPERNOVA trial, stated: “COVID-19 still poses a significant and disproportionate risk to immunocompromised patients, often leading to severe and prolonged illness. By administering infection-fighting antibodies directly to patients who frequently have inadequate responses to vaccines, the data indicate that sipavibart could provide critical protection against COVID-19 for this highly susceptible group.”
Iskra Reic, Executive Vice President of Vaccines and Immune Therapies at AstraZeneca, added: “Immunocompromised patients currently have few or no options for COVID-19 protection and continue to bear a significant disease burden despite often being fully vaccinated. Sipavibart has the potential to prevent COVID-19 in the immunocompromised, and we will now collaborate with regulatory authorities worldwide to make sipavibart available to these vulnerable individuals.”
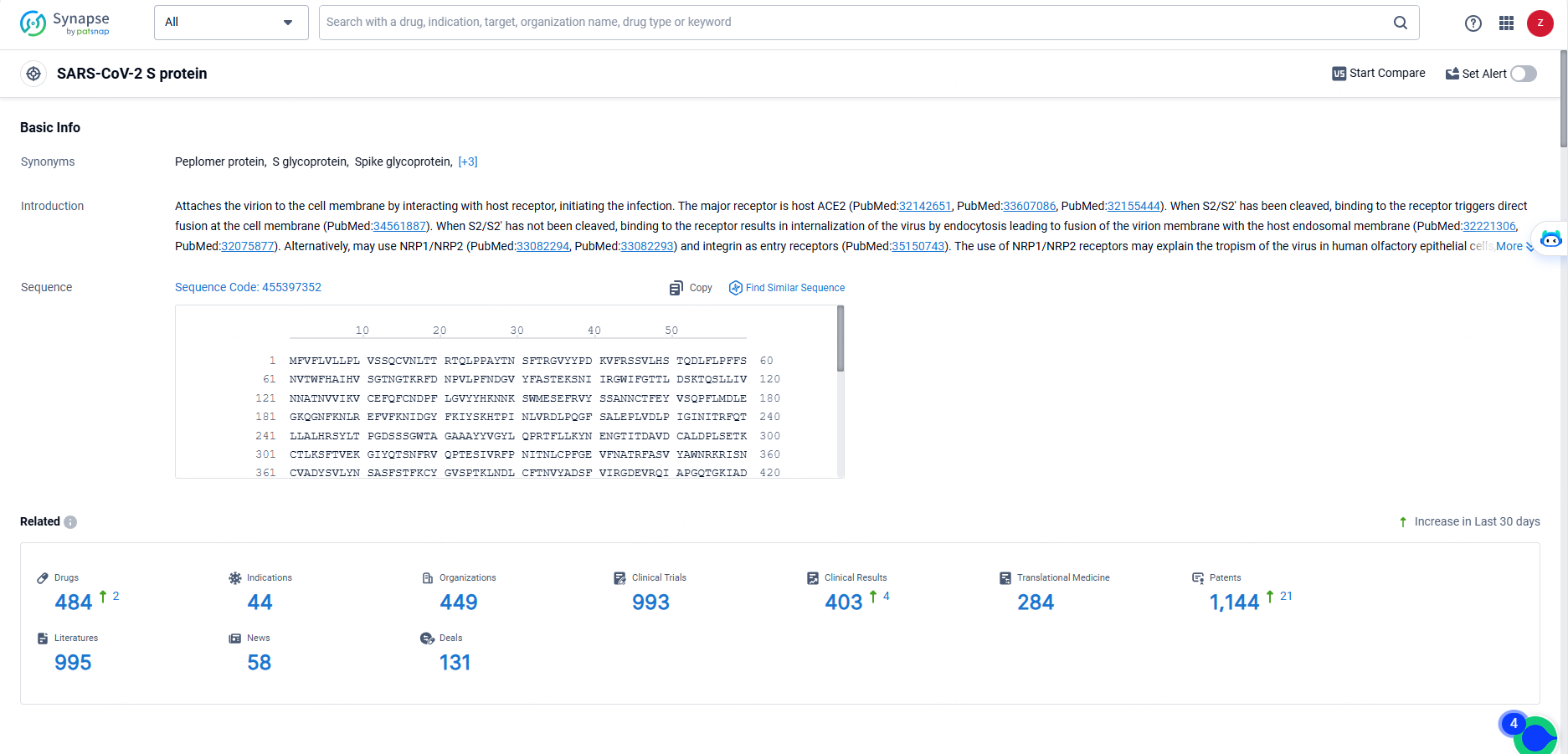
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 23, 2024, there are 484 investigational drugs for the SARS-CoV-2 S protein targets, including 44 indications, 449 R&D institutions involved, with related clinical trials reaching 993, and as many as 1144 patents.
Sipavibart is a monoclonal antibody drug developed by AstraZeneca PLC, targeting the SARS-CoV-2 S protein for the treatment of COVID-19. With its focus on Infectious Diseases and Respiratory Diseases, the drug has reached the Phase 2/3 stage of clinical development, signifying its advancement in the evaluation process. As the global community continues to seek effective solutions for the COVID-19 pandemic, the progress of Sipavibart represents a significant development in the field of biomedicine.