Tagrisso gets EU approval for patients with inoperable EGFR-mutant lung cancer
AstraZeneca's Tagrisso (osimertinib) has received approval from the European Union (EU) for treating adult individuals with locally advanced, unresectable non-small cell lung cancer (NSCLC) that harbors epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations, provided that their condition has not worsened during or after platinum-based chemoradiation therapy (CRT).
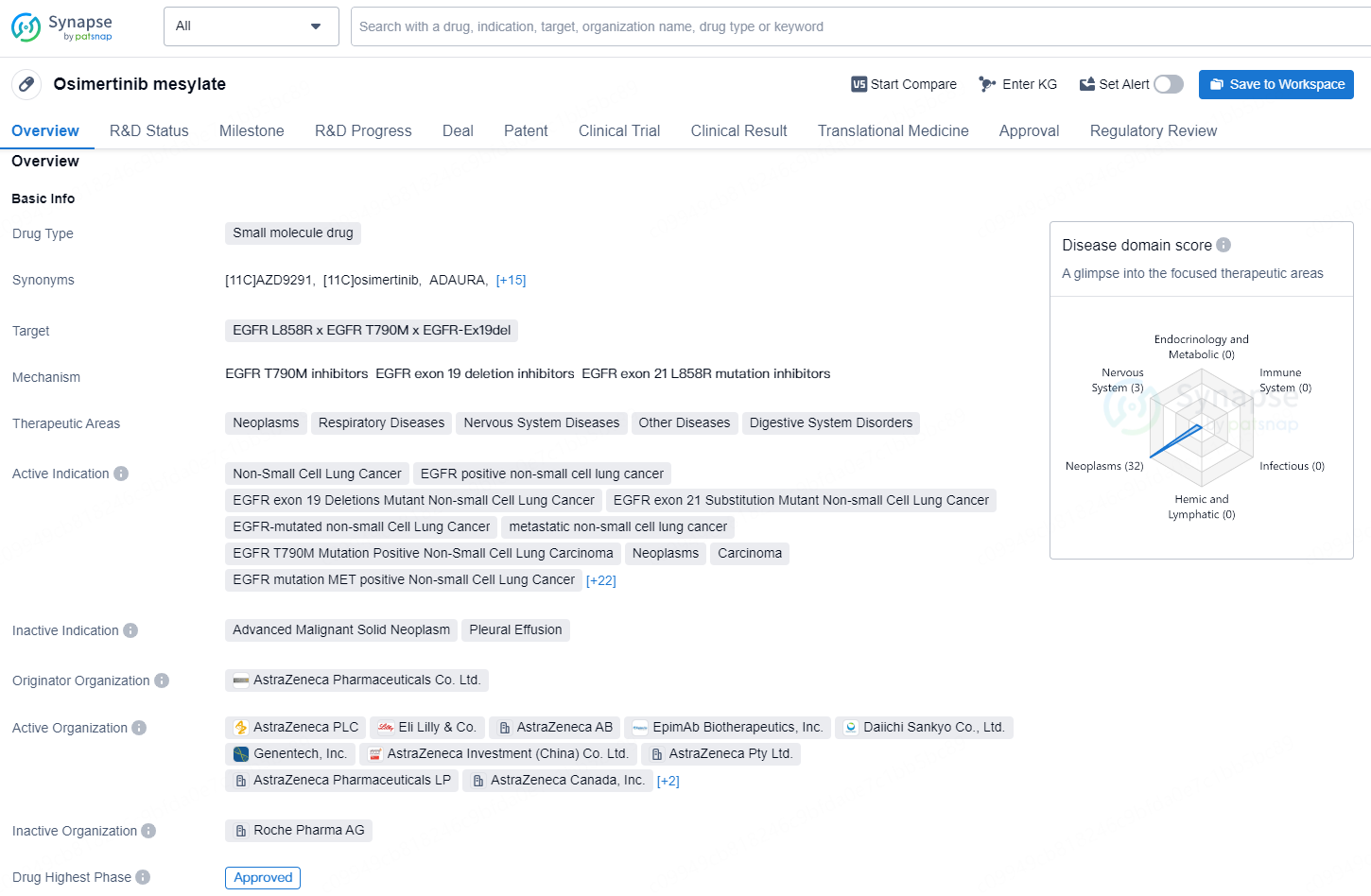
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The European Commission’s approval comes after a favorable recommendation from the Committee for Medicinal Products for Human Use and is grounded in findings from the LAURA Phase III study, which were featured in The New England Journal of Medicine.
In this study, Tagrisso demonstrated an 84% reduction in the risk of disease progression or death when compared to placebo (hazard ratio 0.16; 95% confidence interval 0.10-0.24; p<0.001), as evaluated by a blinded independent central review. The median progression-free survival (PFS) for patients receiving Tagrisso was 39.1 months, whereas it was only 5.6 months for those on placebo.
The overall survival (OS) data remains preliminary, and the study is ongoing to continue evaluating OS as a secondary outcome.
Annually, over 450,000 individuals are diagnosed with lung cancer across Europe, with around 80-85% classified as having non-small cell lung cancer (NSCLC). Of the NSCLC cases in Europe, roughly 10-15% are associated with an EGFR mutation.
Dr. Manuel Cobo, a Specialist Physician in the Medical Oncology Service at Carlos Haya University Hospital in Malaga, Spain, and a trial investigator, stated: “The approval granted today represents a significant advancement for patients in the EU suffering from unresectable, EGFR-mutated non-small cell lung cancer, introducing the first targeted therapy for this condition. In the LAURA trial, osimertinib led to a remarkable 84% reduction in the risk of disease progression or mortality, establishing a novel benchmark for patient outcomes and highlighting the necessity of EGFR mutation testing at the time of diagnosis.”
Dave Fredrickson, AstraZeneca’s Executive Vice President of the Oncology Business Unit, remarked: “Tagrisso is now recognized as the first and only EGFR inhibitor and targeted therapy sanctioned in the EU for locally advanced, unresectable lung cancer, creating a new standard of care for patients who have historically faced rapid disease progression following chemoradiation therapy. The impressive findings from the LAURA trial illustrate that Tagrisso enhances outcomes for patients in the unresectable context, emphasizes the urgency of timely EGFR testing, and positions Tagrisso as the cornerstone therapy for EGFR-mutated non-small cell lung cancer.”
The safety profile of Tagrisso observed in the LAURA trial aligned with its established tolerability, and no new safety issues were reported.
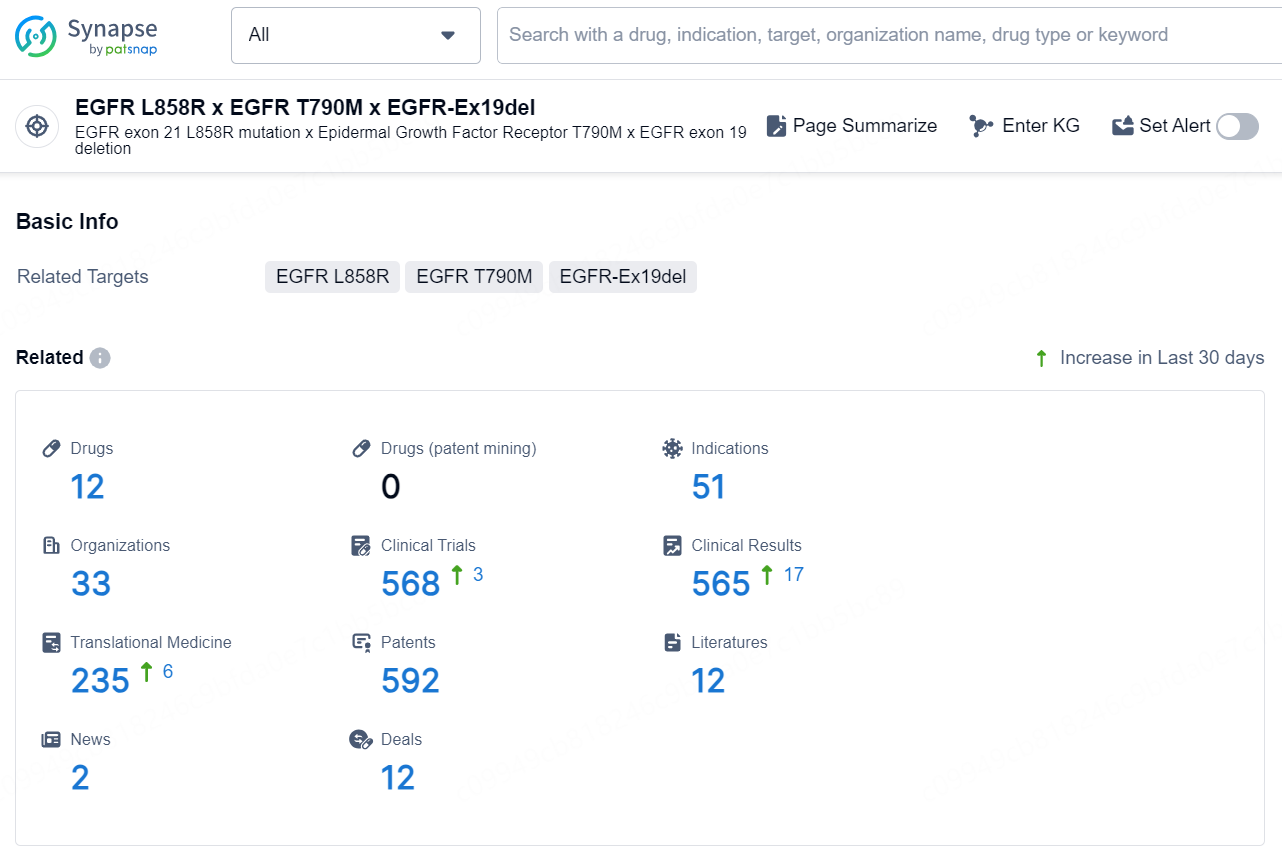
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 30, 2024, there are 12 investigational drug for the EGFR L858R x EGFR T790M x EGFR-Ex19del targets, including 51 indications, 33 R&D institutions involved, with related clinical trials reaching 568, and as many as 592 patents.
Osimertinib mesylate is a small molecule drug developed by AstraZeneca Pharmaceuticals Co. Ltd. It targets the EGFR L858R, EGFR T790M, and EGFR-Ex19del mutations, and is used in the treatment of a wide range of neoplastic and respiratory diseases, as well as nervous system and digestive system disorders.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!