FDA Approves Needle-Free Neffy (Epinephrine Nasal Spray) for Type I Allergic Reactions and Anaphylaxis
ARS Pharmaceuticals, Inc. (Nasdaq: SPRY), a biopharmaceutical firm focused on aiding at-risk individuals and their caregivers in safeguarding patients from severe allergic reactions, which can lead to anaphylaxis, has recently achieved a milestone with the U.S. Food and Drug Administration (FDA) approval of neffy® (epinephrine nasal spray) 2 mg. This new treatment is approved for managing Type I Allergic Reactions, including anaphylaxis, in both adults and children who weigh 30 kg (66 lbs.) or more. This approval marks the first major advancement in epinephrine administration methods in over 35 years and introduces the exclusive needle-free treatment option for individuals and families facing severe allergic conditions.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"Up until now, patients suffering from severe allergic reactions, such as anaphylaxis, had only one treatment option: the often painful and anxiety-inducing epinephrine needle injection. This sometimes led patients to delay or avoid taking the life-saving treatment at the onset of symptoms, thereby increasing the risk of severe reactions or negative outcomes that necessitate further emergency medical intervention," remarked Thomas B. Casale, M.D., Professor of Medicine and Pediatrics and Chief of Clinical and Translational Research in the USF Health Morsani College of Medicine's Division of Allergy and Immunology at the University of South Florida in Tampa, Florida. "With the FDA's approval of neffy, patients with severe allergies now have access to a long-awaited, needle-free, portable method of epinephrine delivery. This has the potential to reduce the time to administration, leading to improved clinical outcomes and enhanced quality of life for both patients and their caregivers."
Type I allergic reactions, triggered by factors such as food, medications, and insect bites, can result in life-threatening anaphylaxis and contribute to approximately 500,000 emergency room visits annually. Reports indicate that nearly 60% of these patients had not received epinephrine before arriving at the ER.
“This approval signifies a pivotal moment in addressing a significant medical need for individuals with Type I allergies by offering an alternative treatment that avoids the use of a needle, which can cause considerable anxiety and fear for many,” stated Richard Lowenthal, Co-Founder, President, and Chief Executive Officer of ARS Pharmaceuticals. “Epinephrine treatment is effective only if it is accessible, straightforward to use, and administered correctly. Our team has dedicated their efforts to developing a compact, easy-to-use, needle-free device that provides reassurance to patients and caregivers by allowing quick and confident epinephrine administration when required. We appreciate the partnership and support of FDA staff in the development of neffy and are profoundly thankful to the severe allergy community — including advocates, patients, parents, and healthcare professionals — for their pivotal role in the development of this essential, life-saving treatment."
The approval of neffy is backed by data from five principal registration studies using a 2 mg intranasal dose of epinephrine. These primary trials were supplemented by various pilot and supportive studies. Neffy met all specified clinical endpoints, and its pharmacokinetic (PK) and pharmacodynamic (PD) data were consistent with approved epinephrine injection products. These data encompassed single and repeated dosing studies in healthy adults, self-administration and caregiver administration in patients with Type I allergies, pediatric patients weighing ≥30 kg (66 lbs.), as well as those with allergic rhinitis. Adverse events in neffy clinical trials were generally mild with no significant nasal irritation or pain, and no serious adverse events were reported.
"Anyone who has experienced or witnessed an anaphylactic reaction understands the stress involved in determining when to self-inject epinephrine or administer it to a child, often causing delays," said Dr. Jonathan Spergel, Chief of the Allergy Program at Children's Hospital of Philadelphia. "We recognize that earlier administration is better, and for many, the needle acts as a barrier that induces dangerous hesitation. Thus, the field has long sought an effective treatment approach that eliminates the need for an injection."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
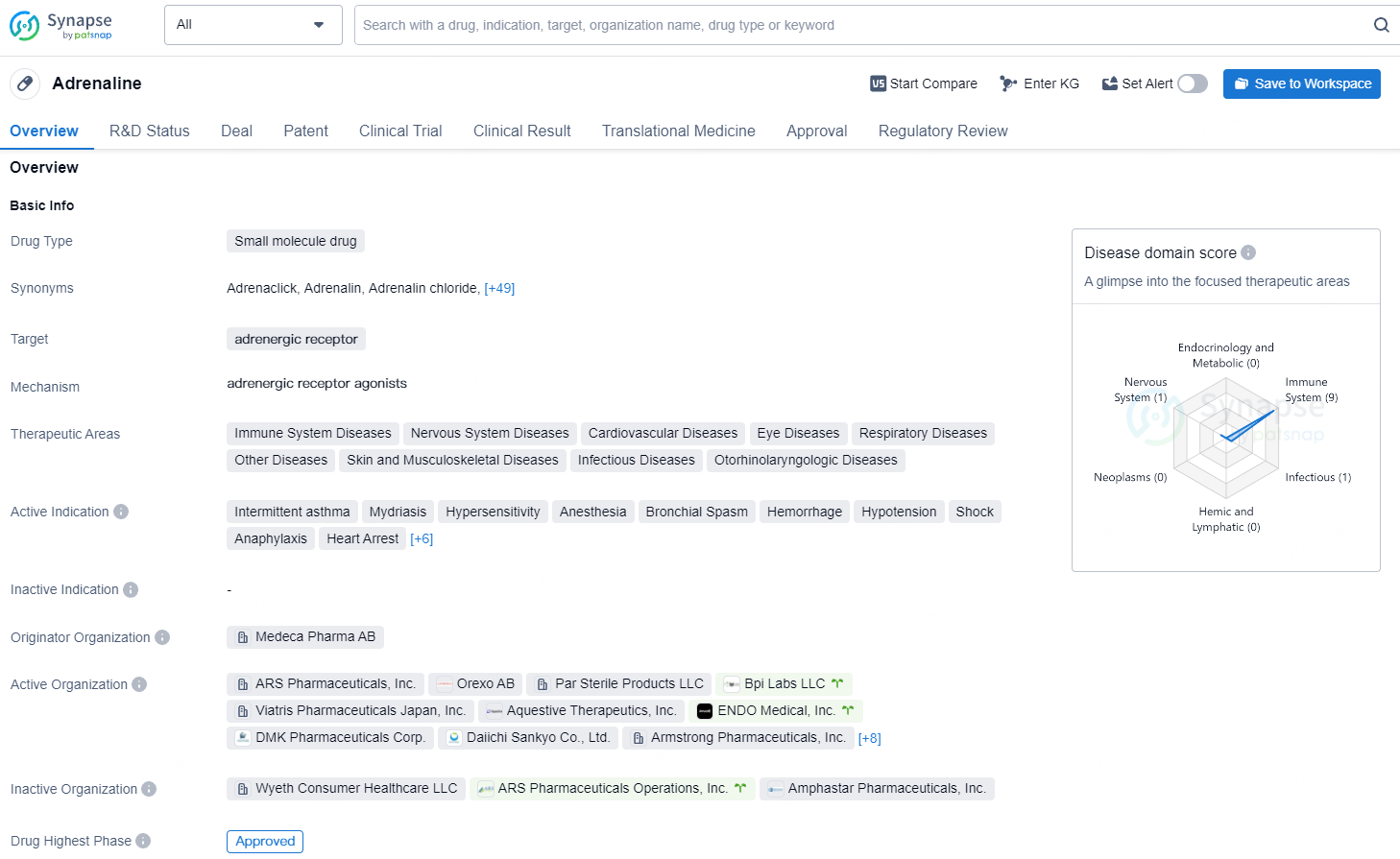
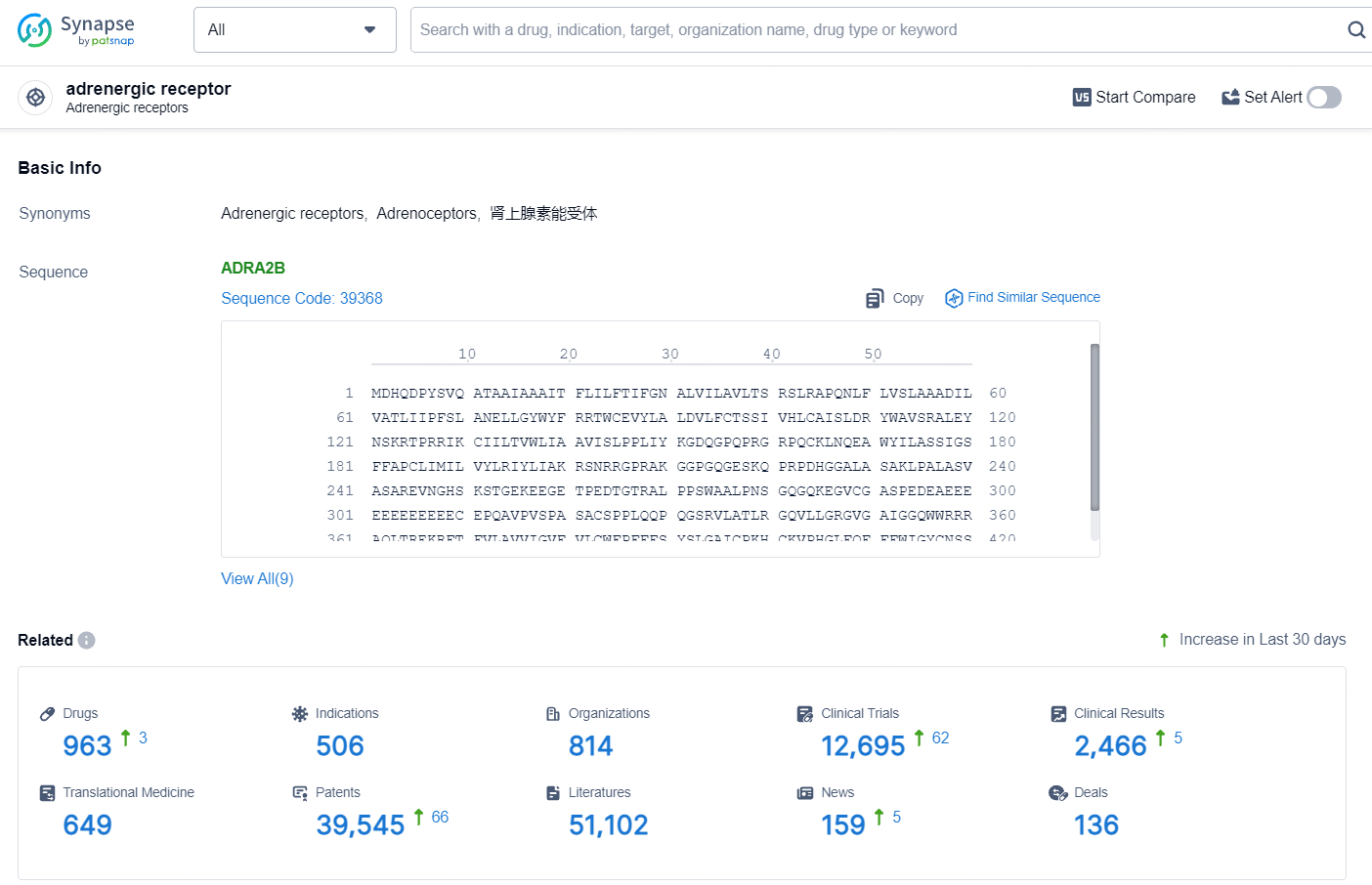
According to the data provided by the Synapse Database, As of August 13, 2024, there are 963 investigational drugs for the adrenergic receptor target, including 506 indications, 814 R&D institutions involved, with related clinical trials reaching 12695, and as many as 39545 patents.
neffy®, also known as epinephrine, is a small molecule drug that targets the adrenergic receptor. It has been approved for use in a wide range of therapeutic areas including immune system diseases, nervous system diseases, cardiovascular diseases, eye diseases, respiratory diseases, skin and musculoskeletal diseases, infectious diseases, and otorhinolaryngologic diseases. The drug is indicated for the treatment of intermittent asthma, mydriasis, hypersensitivity, anesthesia, bronchial spasm, hemorrhage, hypotension, shock, anaphylaxis, heart arrest, oral allergy syndrome, persistent asthma, urticaria, drug hypersensitivity, immediate hypersensitivity, and allergic rhinitis..