The commencement of Phase III SAR-bisPSMA by Clarity and PSI has been initiated
Clarity Pharmaceuticals, a radiopharmaceutical firm in the clinical phase aiming to create advanced solutions that enhance treatment results for pediatric and adult cancer patients, along with PSI CRO AG, an international contract research organization dedicated to timely enrollment in radiopharmaceutical clinical studies, have initiated an agreement and begun working on Clarity's Phase III diagnostic trial for SAR-bisPSMA involving prostate cancer applicants.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The CLARIFY study, stemming from "Positron Emission Tomography using 64Cu-SAR-bisPSMA in individuals with high-risk prostate cancer before radical prostatectomy: A forward-looking, one-branch, multi-site, blinded-review, Phase III diagnostic performance investigation", is a non-randomised, open-label analysis that focuses on 383 subjects.
The main objective of the Phase III study is to determine the diagnostic effectiveness of 64Cu-SAR-bisPSMA PET in identifying prostate cancer in lymph nodes situated within the pelvic area. The examination will be carried out over two imaging periods on days 1 and 2. The initiation of participant enlistment for CLARIFY is planned to occur towards the end of 2023.
Dr Alan Taylor, Executive Chairperson of Clarity made a statement saying, "The anticipation is immense as we edge closer to launching our inaugural registrational Phase III study. With the recent constructive advice received from the US FDA linked to our 64Cu-SAR-bisPSMA program, we eagerly anticipate beginning participant enlistment for the CLARIFY study. We aim to accumulate further information on this next-gen product to further corroborate the convincing preclinical and clinical trial findings to date.
Rhonda Critchlow, Senior Director of Operations at PSI, stated, "Utilising our worldwide repository of more than 400 radiopharmaceutical locations, we can pinpoint those with the optimum resources and abilities for the CLARIFY study. We look forward to partnering with Clarity and concentrating on initiating high-functioning sites to allow rapid recruiting of the first patient."
"Through the various clinical, logistical, and manufacturing advantages that Clarity's Targeted Copper Theranostics platform provides, we are hopeful of enhancing patient treatment results for those suffering from cancer and are committed to achieving this crucial objective," added Rhonda Critchlow.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
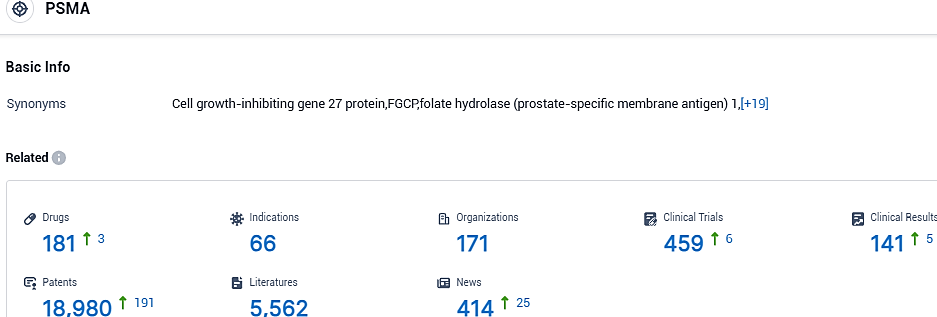
According to the data provided by the Synapse Database, As of October 31, 2023, there are 181 investigational drugs for the PSMA target, including 66 indications, 171 R&D institutions involved, with related clinical trials reaching 459,and as many as 18980 patents.
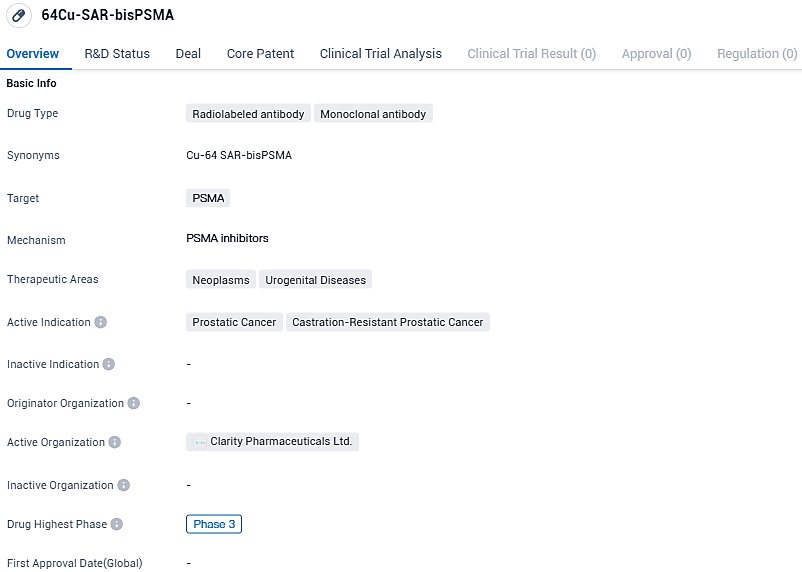
64Cu-SAR-bis PSMA, a molecularly engineered antibody labeled with radiation, is under development for combatting neoplasms and urogenital disorders, particularly prostate cancer and hormone-refractory prostate cancer. At present, it is in the third phase of clinical trials, reflecting its progressed state of assessment. This drug has a distinct mode of action that could potentially enhance therapeutic results for individuals dealing with prostate cancer.