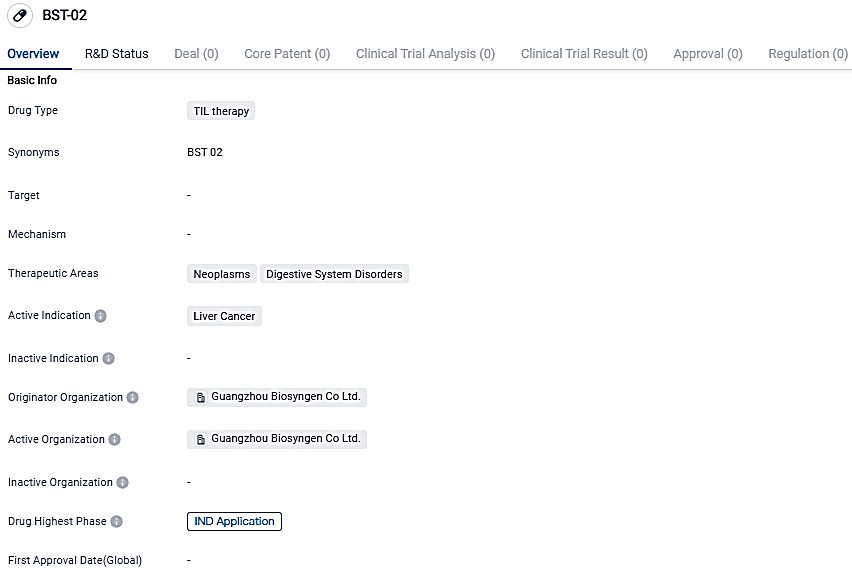
The FDA gives IND approval for Biosyngen's BST02, the first global TIL treatment for liver cancer
The US FDA has approved Biosyngen's BST02, a TIL therapy for liver cancer, for clinical trials. BST02 is an innovative product in the cell and gene therapy sector, marking the first worldwide TIL therapy developed for the treatment of all liver cancer varieties to advance to clinical phases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
TIL therapy operates through harvesting naturally occurring lymphocytes within a tumor, these cells are then expanded in a controlled lab setting to improve their functionality. Thereafter, these enhanced lymphocytes are reintroduced to the patient.
TIL therapy presents several key benefits. These comprise the availability of various TCR clones, improved tumor targeting ability, and minimized targeted toxicity. Accordingly, TIL therapy offers considerable hope and advantages in curing solid tumors.
The endorsement of BST02 serves as yet another notable accomplishment for Biosyngen as the fourth groundbreaking product in the company's portfolio to secure an IND. This milestone achievement comes as a result of employing Biosyngen's unique and impactful global integrated R&D translational model.
Biosyngen has secured IND authorization in both China and the United States for four pioneering products within the past nine months. This feat reaffirms the company's standing as a rising biotech with internal R&D capabilities, excelling in CAR-T, TCR-T and TIL therapies within T cell therapy domain. These tumor-specific T cells therapies, namely CAR-T, TCR-T, and TIL mark a substantial progression in treating solid tumors.
Even though these therapies follow a common development path, their technological methodologies and production process can differ. At present, Biosyngen has built comprehensive technology platforms and repositories inclusive of IDENTIFIER®, these act as a sturdy base for discovering and identifying antigens, antibodies, and TCR, besides facilitating the design of a range of specialized therapeutic products.
Through steady, continuous and sustainable endeavors, Biosyngen can answer unmet clinical needs and push the development of innovative immunotherapy medicines of significant value, thereby benefiting patients globally.
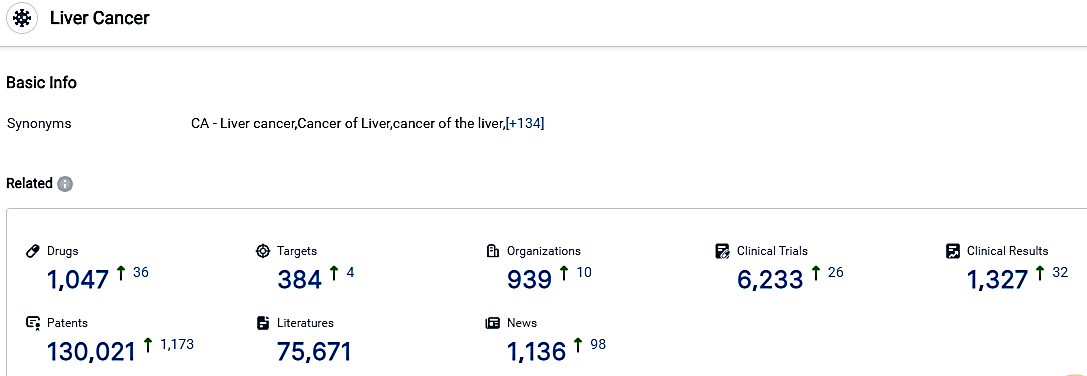
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
According to the data provided by the Synapse Database, As of October 30, 2023, there are 1047 investigational drugs for the Liver Cancer, including 1047 targets, 939 R&D institutions involved, with related clinical trials reaching 6233,and as many as 130021 patents.
BST02 is a newly developed form of adoptive immune cell treatment that utilizes an expanded form of tumor infiltrating lymphocytes, originating from the individual's own cells. The therapy was created uniquely for combating liver cancer types, such as hepatocellular carcinoma and cholangiocarcinoma. By implementing cryopreservation, BST02 has surmounted previously identified limitations – it solves the issues related to geographic distance and relinquishes the requirement for substantial quantities of interleukin-2. Initial results from preliminary clinical tests have provided indications of its security and effectiveness.