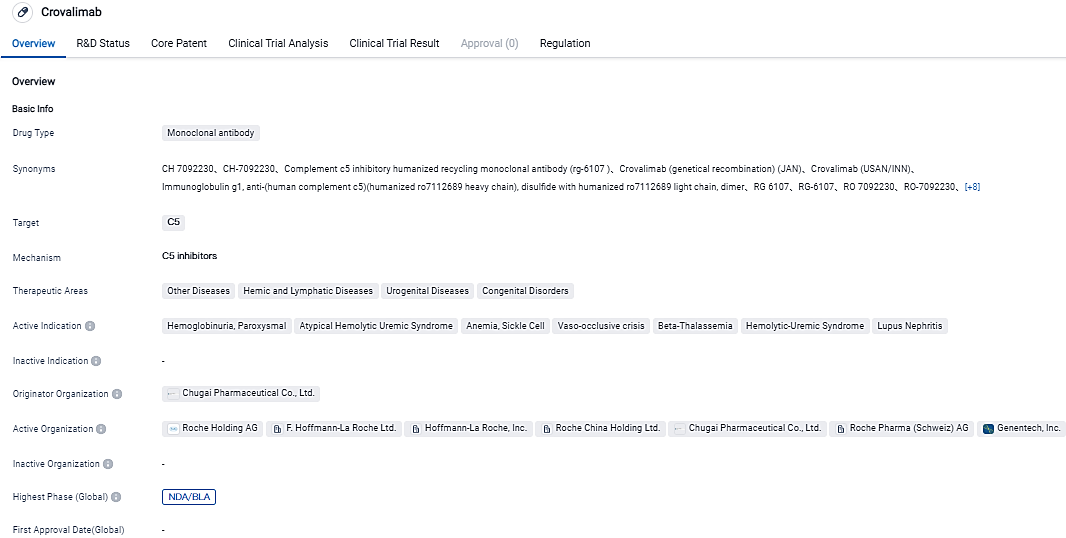
The FDA accepts Genentech's Crovalimab application designed to address PNH, an uncommon but potentially fatal blood disorder
Genentech, part of the Roche Group, has revealed that the FDA has accepted to their BLA for crovalimab, an exploratory, newly-developed anti-C5 recycling monoclonal antibody, meant to treat paroxysmal nocturnal hemoglobinuria. The endorsement was given based on the outcomes from the decisive Phase III COMMODORE 2 study, where crovalimab showed effective disease management and was well-received. Findings from the Phase III COMMODORE 1 analysis, showcasing the steady advantage-risk ratio of crovalimab, added weight to the submission.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
“This acceptance of the filing underlines the value of crovalimab, specifically designed to be reused within the bloodstream, aiming to provide a long-lasting effect while minimizing treatment impact,” said Levi Garraway, M.D., Ph.D., the principal medical officer and Global Product Development Lead. “Crovalimab has the potential to offer self-administration as rarely as every four weeks, thereby lessening clinical visits for individuals living with this enduring disorder.”
PNH is an uncommon and potentially fatal blood disorder, impacting around 20,000 individuals globally. PNH involves the destruction of red blood cells by the complement system, an integral part of the innate immunity. This leads to issues like anemia, lethargy, and blood clots, and can further lead to renal disease. C5 inhibitors – therapies that hinder parts of the complement system chain – have demonstrated effectiveness in treating PNH.
The BLA was based on results from the Phase III COMMODORE 2 trial featuring subjects with PNH not previously treated with complement inhibitors. The study found that crovalimab, delivered as SC injections every four weeks, controlled the disease and was as effective and safe as eculizumab, a current standard-of-care, provided intravenously biweekly.
Adverse events happened in 78% of participants who treated crovalimab, and 80% treated eculizumab. The request included data from the Phase III COMMODORE 1 trial, bolstering the favourable benefit-risk ratio of crovalimab in PNH patients transitioning from current C5 inhibitors. Information from the COMMODORE 1 and 2 trials were shared recently at the European Hematology Association 2023 Hybrid Congress.
Global Phase III data from the COMMODORE 1 and 2 trials in PNH have been submitted to various regulatory entities globally, and the submission process continues. Crovalimab is being investigated extensive clinical development scrutiny, encompassing five ongoing Phase III trials and three earlier stage trials in PNH as well as other complement mediated disorders.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
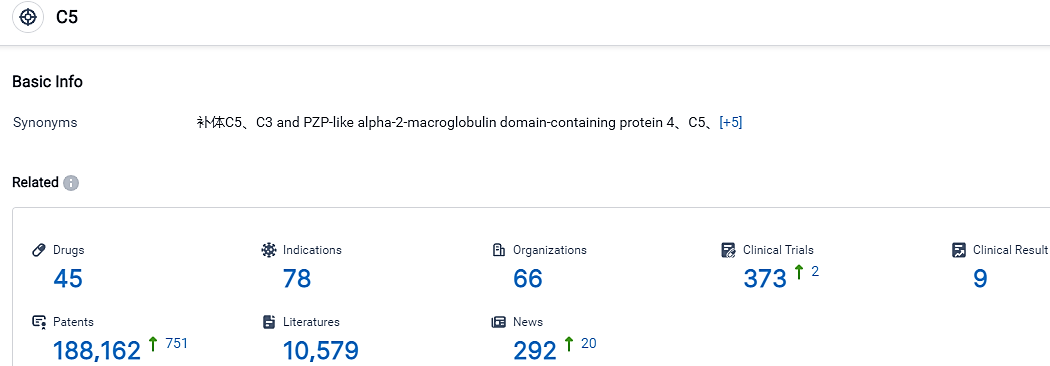 According to the data provided by the Synapse Database, As of September 7, 2023, there are 45 investigational drugs for the C5 target, including 78 applicable indications,66 R&D institutions involved, with related clinical trials reaching 373,and as many as 188162 patents.
According to the data provided by the Synapse Database, As of September 7, 2023, there are 45 investigational drugs for the C5 target, including 78 applicable indications,66 R&D institutions involved, with related clinical trials reaching 373,and as many as 188162 patents.
Crovalimab, which was created by Chugai Pharmaceutical Co., Ltd, has been engineered to address certain needs of people living with complement-mediated diseases, including providing patients with a potential self-administration option. crovalimab binds to a different C5 binding site from current treatments. It is also being evaluated in atypical hemolytic uremic syndrome, sickle cell disease, and other complement mediated diseases.