The FDA expedites reviewing Merck's request to use KEYTRUDA®, alongside chemoradiotherapy, for cervical cancer treatment
Merck, also recognized as MSD beyond the US and Canada, has reported that the U.S. FDA is conducting a priority analysis on a fresh sBLA petitioning for authorization of KEYTRUDA, Merck’s anti-PD-1 treatment. This is intended for use alongside external beam radiotherapy and simultaneous chemotherapy, succeeded by brachytherapy, as a definitive remedy for newly identified patients struggling with high-risk locally advanced cervical cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The sBLA is derived from findings in the KEYNOTE-A18 study, also referred to as ENGOT-cx11/GOG-3047, where the combination of KEYTRUDA and concurrent chemoradiotherapy showed a statistically and clinically substantial enhancement in PFS as opposed to just concurrent chemoradiotherapy. If authorized, this would mark Merck's third approved application in cervical cancer and the first at an earlier stage of the illness. The FDA has established a Prescription Drug User Fee Act, or action due date, to January 20, 2024.
"For over 20 years, there has been no significant change in the treatment for locally advanced cervical cancer patients, and most patients often witness a recurrence or their disease advancing," stated Dr. Gursel Aktan, VP of global clinical development, Merck Research Laboratories. "Upon approval, KEYTRUDA will become the maiden immunotherapy for newly diagnosed high-risk locally advanced cervical cancer patients."
In the United States, KEYTRUDA has dual approved uses in cervical cancer: it can be combined with chemotherapy, optionally with bevacizumab, as a treatment for persistent, recurrent, or metastatic cervical cancer where tumors showcase PD-L1 as confirmed through an FDA-approved analysis and as a solitary agent and in situations where patients with recurrent or metastatic cervical cancer whose condition progresses during or post-chemotherapy have tumors that express PD-L1, as confirmed by an FDA-approved analysis.
Merck is progressing research seeking to enhance results for patients affected by breast and gynecological cancers. Merck's extensive clinical development program covers more than 15 Merck-led Phase 3 trials evaluating KEYTRUDA as a standalone therapy or in combination with other medications. Currently, in the U.S., there are two approved uses of KEYTRUDA for triple-negative breast cancer and another four approved uses for gynecological cancers.
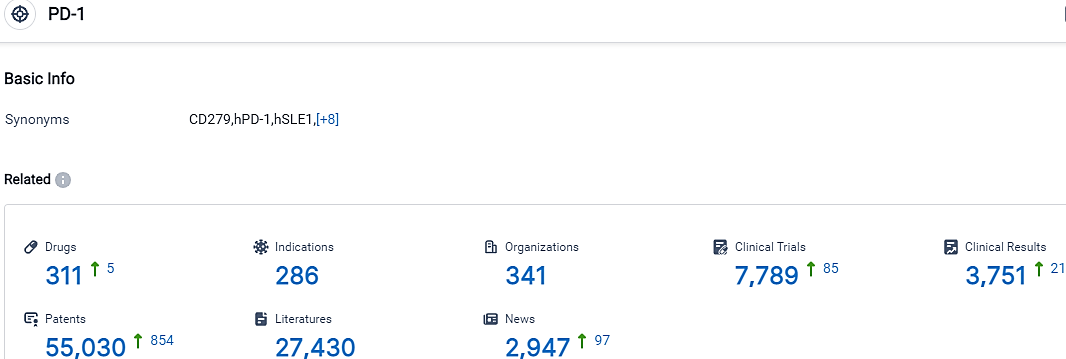
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 26, 2023, there are 311 investigational drugs for the PD-1 target, including 286 indications,341 R&D institutions involved, with related clinical trials reaching 7789,and as many as 55030 patents.
KEYTRUDA functions as an anti-programmed death receptor-1 treatment, bolstering the body's immune system's capacity to identify and combat cancer cells. The clinical program for KEYTRUDA is aimed towards grasping its functionality across diverse cancer types and determining parameters that might indicate a patient's potential to respond positively to KEYTRUDA treatment, which entails investigating a range of distinct biomarkers.