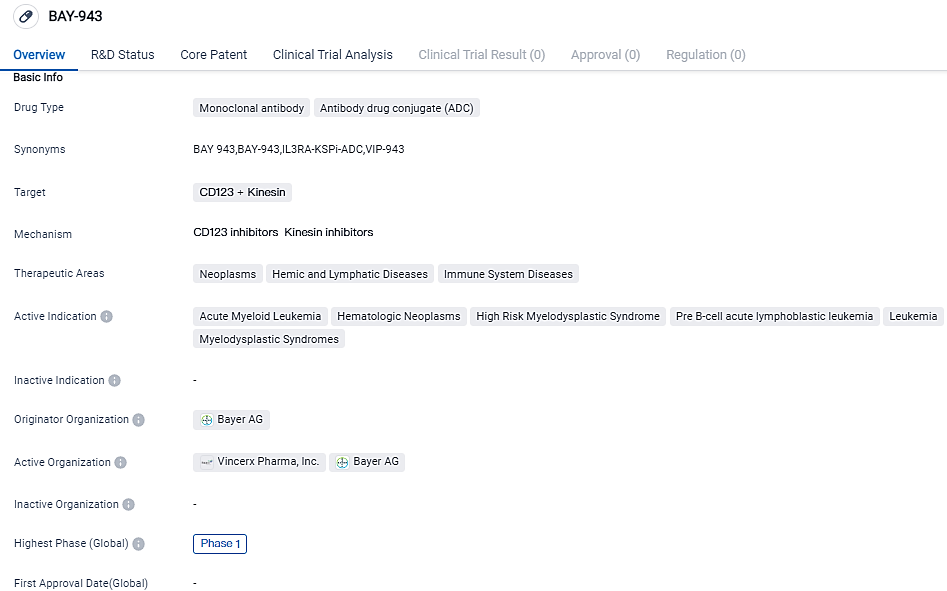
The first patient of Vincerx Pharma's Phase 1 Clinical Tria of VIP943 has been administered
Vincerx Pharma, Inc., a bio-pharmaceutical company aiming to address the medical requirements of cancer patients by revolutionizing the therapeutic approaches, declared that the initial patient in the Phase 1 clinical trial has received treatment with VIP943. This trial focuses on patients with recurring or resistant cases of acute myeloid leukemia, myelodysplastic syndrome, and B-cell acute lymphoblastic leukemia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"The initial administration to our first patient marks a significant progress for the VIP943 development plan and our advanced bioconjugation platform. This achievement occurred 17 business days from receiving our 'safe to proceed' notice, highlighting the dedication of our research team and the operational excellence of the Vincerx team," explained Ahmed Hamdy, M.D., CEO of Vincerx.
"VIP943 is the first ADC product derived from bioconjugation technique, purposely configured to solve the challenges encountered with conventional ADCs. This unique and proprietary blend has produced robust preclinical signs of efficacy and safety, bringing about a potential evolution in treating CD123+ hematologic malignancies. We're eager to assess VIP943's potential and anticipate revealing preliminary safety and pharmacological findings from our Phase 1 trial in 2024," added Ahmed Hamdy, M.D.
Stephen A. Strickland, Jr., M.D., MSCI, Director of Leukemia Research at the Sarah Cannon Research Institute, commented, "Numerous factors affect the prognosis of these cancers, like higher prevalence in geriatric individuals and low response levels. Vincerx's preclinical findings provide compelling evidence to endorse the testing of VIP943 on hematologic malignancies. We aim to provide more effective and less toxic therapeutic alternatives for patients."
Howard A. Burris, III, M.D., President of SCRI and Scientific Advisory Board member at Vincerx, stated, "An ADC that couples selective CD123 binding with a potentially superior safety profile, in comparison with other ADCs and CD123-targeting treatments, could offer a desperate need for patient treatment. Our squad is thrilled to cooperate with Vincerx and start patient enrolment in this Phase 1 dose escalation study."
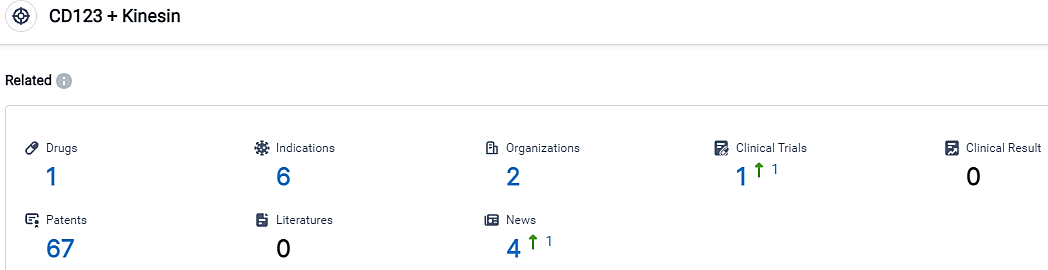
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 19, 2023, there are 1 investigational drugs for the CD123 and Kinesin target, including 6 indications,2 R&D institutions involved, with related clinical trials reaching 1,and as many as 67 patents.
VIP943 is the lead program from our next-generation ADC platform. Once inside the cell, it is only cleaved by an intracellular protein called legumain, allowing specific release and activation of the KSPi payload within the cancer cell. KSPi provides a novel way to deliver a cell cycle arrest agent, leading to cell death, which is a clinically validated ADC payload class mechanism. Further research and development are required to determine its efficacy and safety profile.