Progress in the Research of COMT Inhibitors
Catechol O-methyltransferase (COMT) is an enzyme responsible for the O-methylation of endogenous neurotransmitters, xenobiotics, and hormones with catechol structures. COMT exists in mammalian in two forms: soluble (S-COMT) and membrane-bound (MB-COMT). S-COMT is the main form of COMT in peripheral organs, while MB-COMT is more abundant in the central nervous system. The physiological substrates of COMT include L-dopa, catecholamines (dopamine, norepinephrine, and epinephrine), their hydroxylated metabolites, catechol estrogens, ascorbic acid, and intermediate dihydroxyindoles of melanin.
Specifically, COMT plays a key role in the inactivation and metabolism of dopamine and other catechol compounds. It reduces catechol molecules to prevent genomic damage caused by oxygen radicals formed either through the formation of DNA adducts or through the catechol redox cycle. COMT is a pharmacological target for the treatment of a variety of central and peripheral nervous system diseases, including Parkinson's disease, depression, schizophrenia, and other dopamine-deficiency related diseases.
COMT Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 15 COMT drugs worldwide, from 21 organizations, covering 13 indications, and conducting 179 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
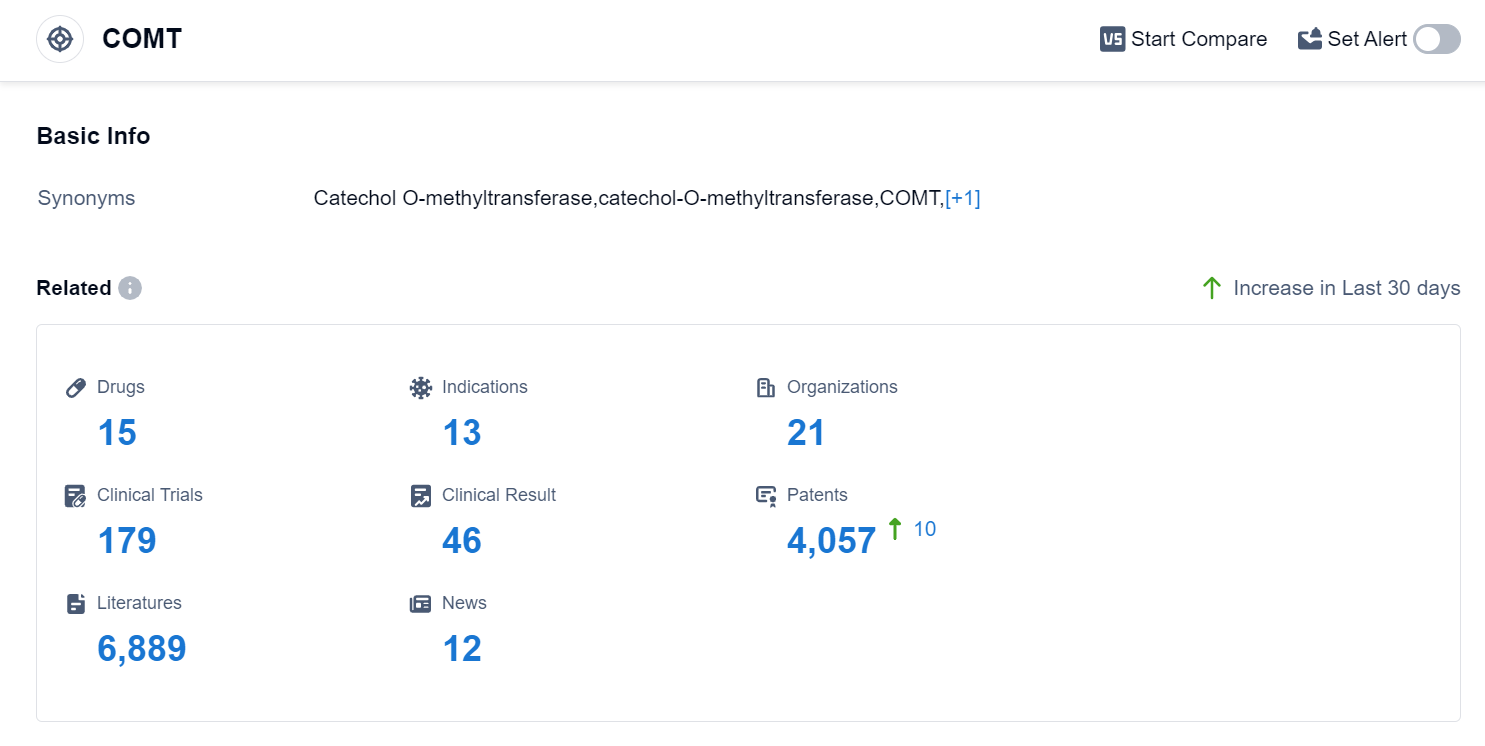 The analysis of the current competitive landscape of target COMT reveals that Orion Pharma Ltd., Orion Oyj, Novartis AG, BIAL-Portela & Cia SA, and Neurocrine Biosciences, Inc. are some of the companies growing fastest under this target. The highest stage of development is the Approved phase, with a significant number of drugs. These drugs have been approved for indications such as Parkinson Disease, Muscle Spasticity, and other neurological disorders. Small molecule drugs are progressing rapidly, indicating intense competition in the market. The European Union, United States, China, and Japan are the countries/locations with the highest development under this target, with China showing significant progress. Overall, the target COMT presents a competitive landscape with multiple companies and drug types, indicating a promising future for the development of drugs targeting this enzyme.
The analysis of the current competitive landscape of target COMT reveals that Orion Pharma Ltd., Orion Oyj, Novartis AG, BIAL-Portela & Cia SA, and Neurocrine Biosciences, Inc. are some of the companies growing fastest under this target. The highest stage of development is the Approved phase, with a significant number of drugs. These drugs have been approved for indications such as Parkinson Disease, Muscle Spasticity, and other neurological disorders. Small molecule drugs are progressing rapidly, indicating intense competition in the market. The European Union, United States, China, and Japan are the countries/locations with the highest development under this target, with China showing significant progress. Overall, the target COMT presents a competitive landscape with multiple companies and drug types, indicating a promising future for the development of drugs targeting this enzyme.
The globally approved COMT inhibitor on the market: Entacapone
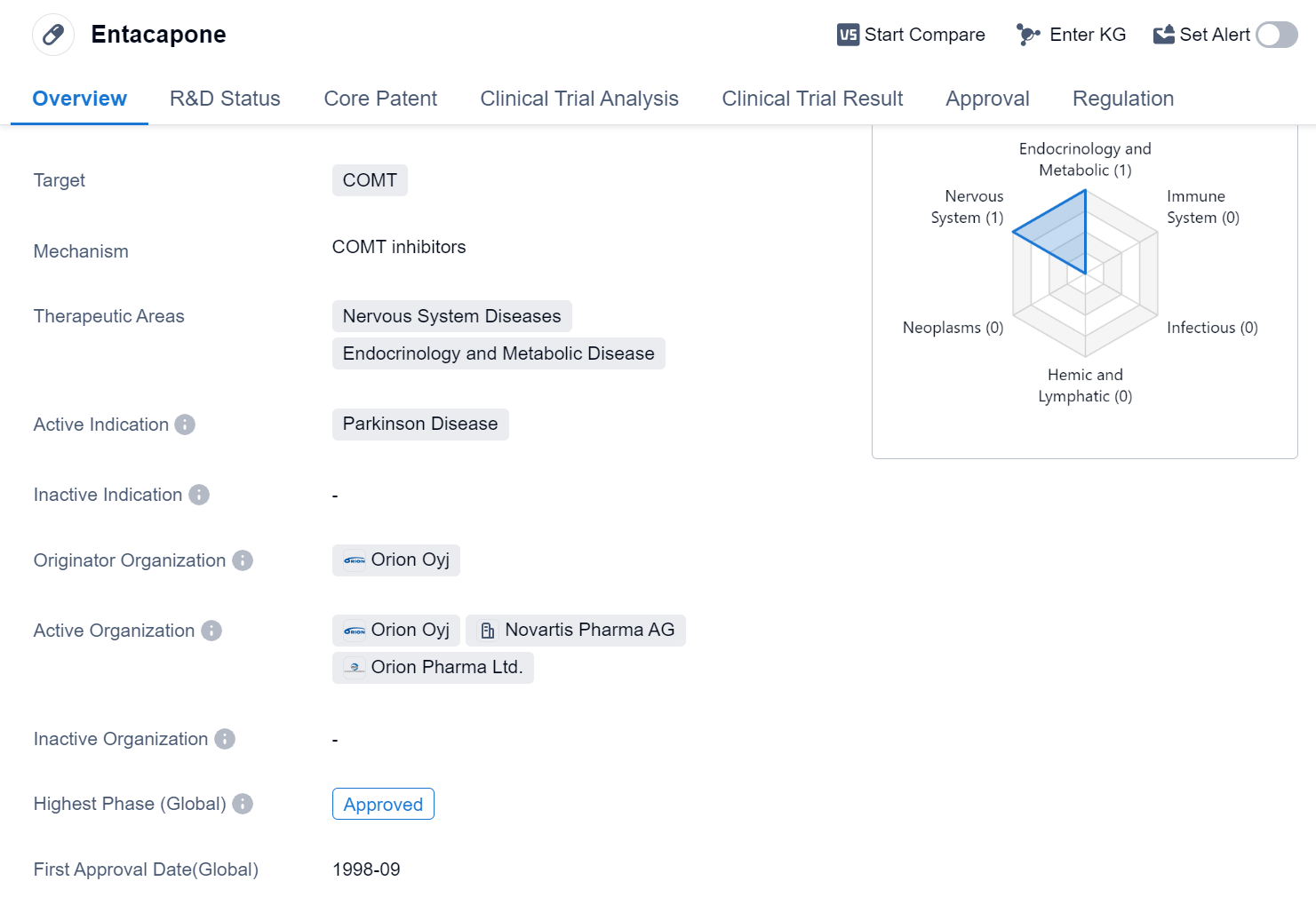
Entacapone is a small molecule drug that targets the enzyme catechol-O-methyltransferase (COMT). It is primarily used in the treatment of Parkinson's disease, a neurodegenerative disorder that affects the nervous system. The drug is classified as a therapeutic agent for nervous system diseases and endocrinology and metabolic diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Entacapone was first approved for use in the European Union in September 1998. It is developed and marketed by Orion Oyj, a pharmaceutical company. The drug has also received approval in China, indicating its global reach and acceptance.
The highest phase of development for Entacapone is approved. This status signifies that the drug has met the necessary regulatory requirements and has been deemed suitable for use in patients.
The regulatory status of Entacapone is classified as priority review. This designation implies that the drug has been given special attention by regulatory authorities due to its potential to address an unmet medical need or provide significant therapeutic benefits. Priority review status expedites the review process, allowing the drug to reach the market faster.
In summary, Entacapone is a small molecule drug developed by Orion Oyj that targets COMT. It is primarily used in the treatment of Parkinson's disease and is classified as a therapeutic agent for nervous system diseases and endocrinology and metabolic diseases. The drug has received approval in both the European Union and China, indicating its global availability. Entacapone has successfully completed clinical trials and has been granted priority review status, highlighting its potential to address unmet medical needs.