The Surge and Impact of Antibody-Drug Conjugates (ADCs) in Modern Oncology
According to a report by Grand View Research, the Antibody-Drug Conjugates (ADCs) market has entered a new phase of rapid growth since 2019, maintaining an annual growth rate exceeding 30%. In 2023, the market size surpassed the $10 billion milestone for the first time, reaching an estimated $11.32 billion. It is projected to grow at a compound annual growth rate (CAGR) of 9.2% from 2024 to 2030, ultimately reaching $27.37 billion by 2033. Notably, Enhertu is expected to achieve an annual sales figure of $3.5 billion in 2024.
Composition and Mechanism of Action of ADCs
As a cutting-edge therapeutic approach, ADCs achieve precise targeting of tumor cells by covalently linking highly cytotoxic drugs (typically small-molecule drugs) with monoclonal antibodies (mAbs). This allows ADCs to utilize the high specificity of monoclonal antibodies to recognize and bind to specific antigens on the surface of tumor cells. The cytotoxic drugs are then selectively delivered to these cells, enabling targeted destruction while minimizing damage to healthy cells. This selectivity has made ADCs a vital component of modern oncology treatments. With ongoing advancements, ADCs are increasingly significant in precision medicine.
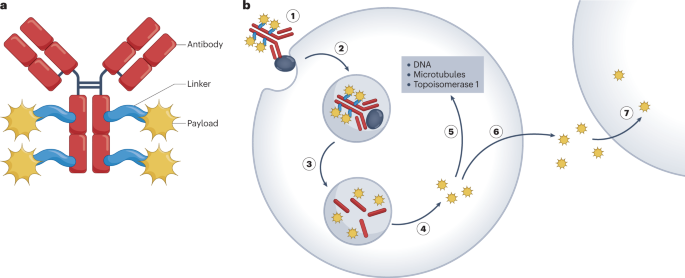
The basic structure of ADCs comprises three key components: the monoclonal antibody, the linker, and the cytotoxic payload. The monoclonal antibody identifies and binds to antigens on tumor cell surfaces. The linker ensures stable attachment of the antibody and cytotoxic drug, preventing premature release before reaching the target cells. Once delivered, the cytotoxic payload is released inside the target cell, often through endocytosis. The payload then disrupts cellular structure or function, leading to apoptosis. For example, the released payload can affect the cell cycle by inhibiting DNA synthesis, interfering with spindle formation, or inducing DNA double-strand breaks, ultimately triggering programmed cell death.
This mechanism not only effectively eradicates tumor cells but also reduces off-target toxicity due to intracellular drug release. Additionally, by fine-tuning the chemical properties of the linker, researchers can design ADCs that remain stable in the bloodstream and release their payload only upon reaching the target cells.
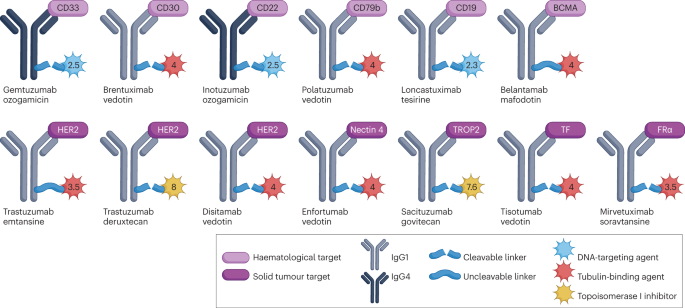
Through such precise targeting, ADCs provide new therapeutic options for difficult-to-treat cancers while minimizing damage to healthy tissues. Furthermore, ongoing innovations in linking technologies and drug carriers are enhancing therapeutic efficacy and significantly reducing side effects during treatment.
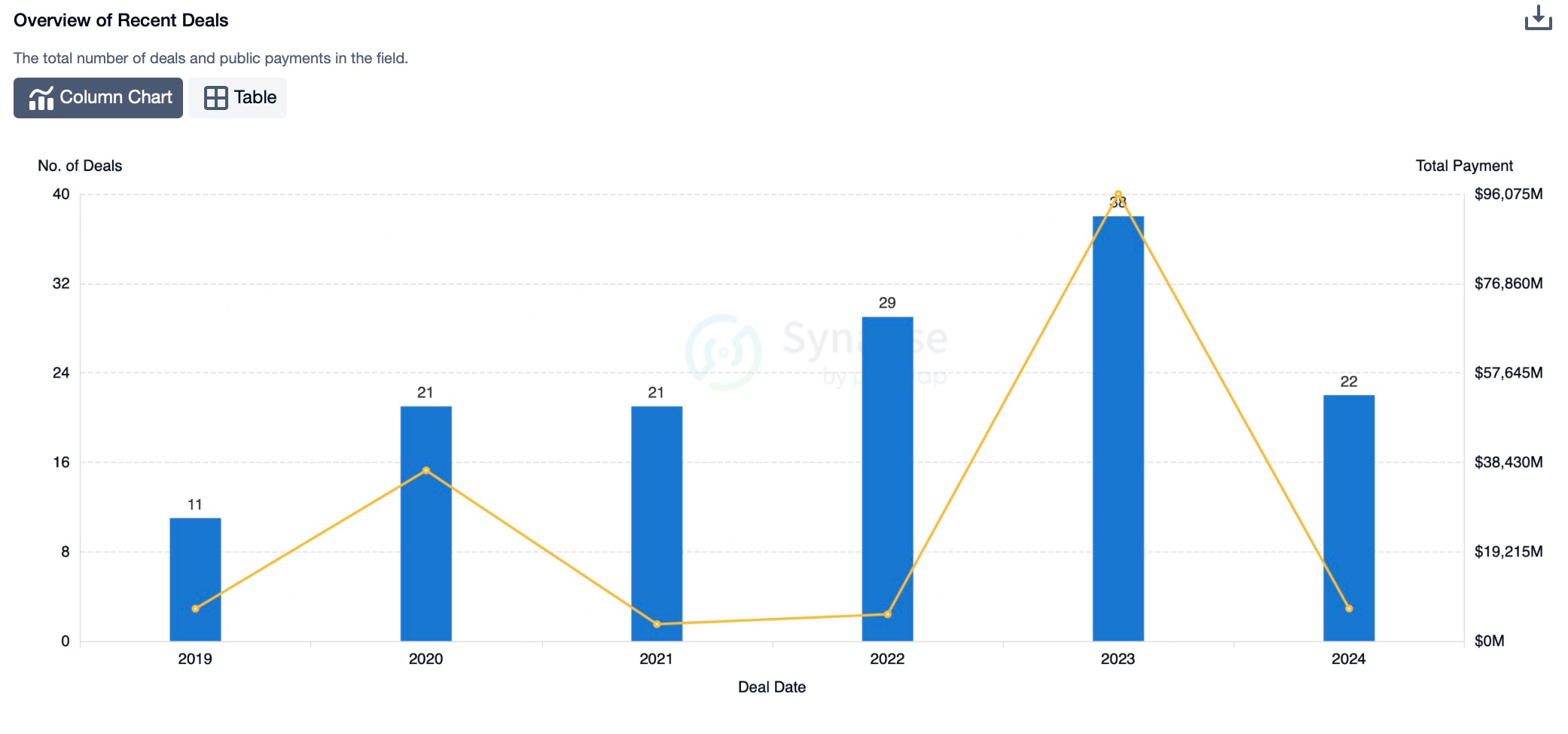
Factors Driving the Growth of the ADC Drug Market
The primary factors driving the growth of the antibody-drug conjugate (ADC) market include the following: First, the ongoing research and development activities in the fields of cancer biology and immunology have deepened our understanding of the tumor microenvironment, enabling scientists to design ADC drugs that are both more efficient and have fewer side effects. Second, the growing global incidence of cancer has increased the demand for innovative treatment modalities. ADCs have garnered attention for their efficacy in addressing cases that are challenging for traditional chemotherapy. Lastly, advancements in drug delivery technologies, particularly the optimization of linker systems, have significantly enhanced the safety and efficacy of ADCs. Additionally, regulatory support for the accelerated approval of ADC drugs and active strategic collaborations among biotechnology companies have greatly facilitated the development and marketization of ADC therapies.
Delivery of ADCs
The delivery of ADCs is a complex and precise process aimed at ensuring that the drug accurately reaches tumor cells and releases its cytotoxic payload. Traditional naked antibodies can be administered directly via intravenous injection and rely on their high affinity for antigens on the surface of tumor cells for targeting. However, their rapid clearance from the body may reduce therapeutic efficacy. To address this limitation, chemical modifications, such as PEGylation (polyethylene glycol conjugation), have been introduced to enhance the stability of ADCs and extend their plasma half-life. This allows ADCs to remain in the bloodstream for a longer duration, increasing the likelihood of reaching tumor sites.
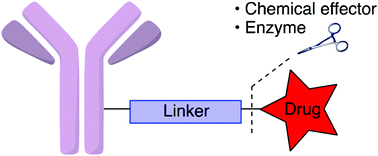
Linker technology is a critical component of ADC design and can be classified into two main categories: cleavable linkers and non-cleavable linkers. Cleavable linkers are designed to release the cytotoxic payload under specific conditions, such as enzymatic activity in the lysosome or changes in pH. Non-cleavable linkers, on the other hand, remain stable within the cell until the drug portion is released, ensuring that the cytotoxic molecules are delivered at the correct time and location.
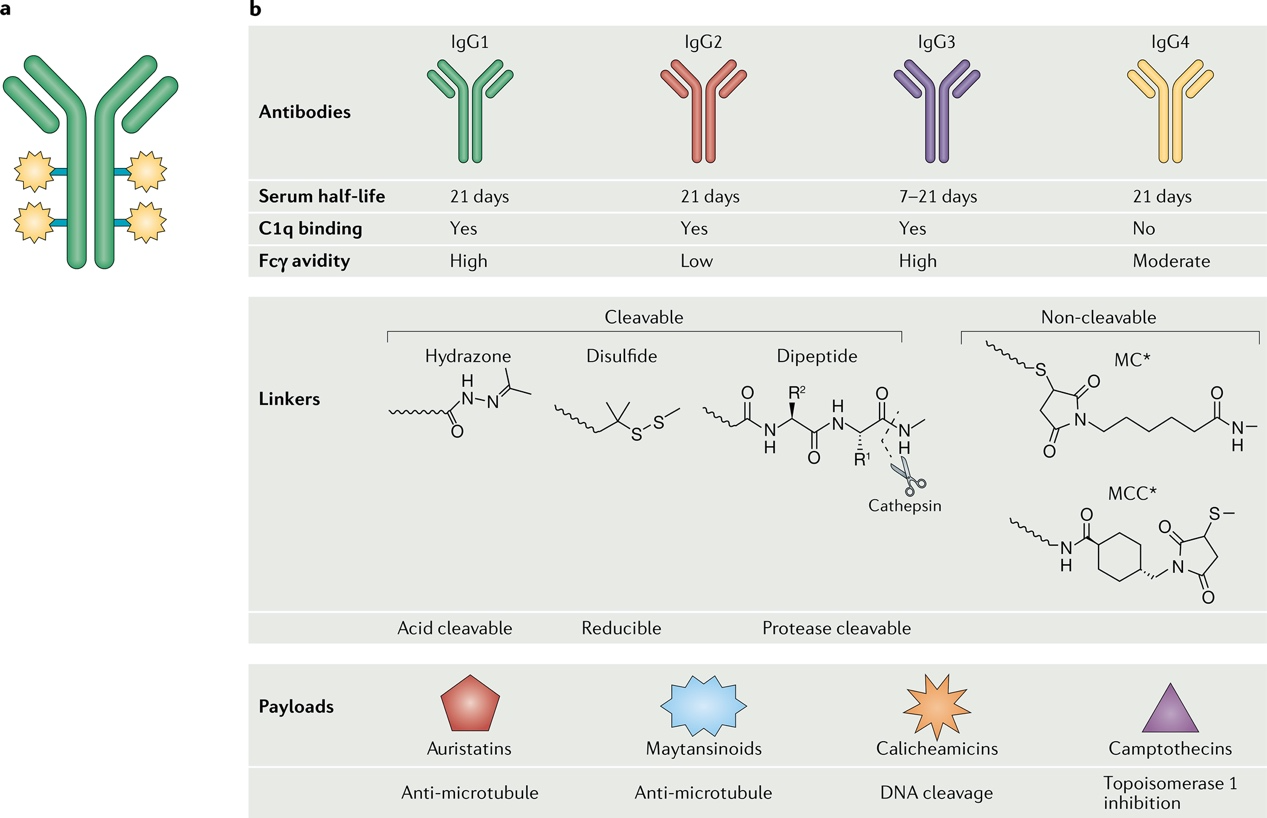
Moreover, nanoparticle technologies like liposomal encapsulation have been employed to protect ADCs from degradation and enhance cellular uptake. Liposomes, as an effective drug carrier system, can transport ADCs across biological barriers and precisely release the drug at target sites, thereby improving drug delivery efficiency. Polymer matrix systems offer another approach to enhancing the stability and delivery efficacy of ADCs. By integrating ADCs into polymer matrices, the physicochemical properties of the drugs, such as solubility and stability, can be improved, optimizing therapeutic outcomes.
The combined application of these delivery strategies can effectively overcome biological barriers, ensuring that ADCs reach tumor cells efficiently and safely while minimizing adverse effects on healthy tissues. With continued innovation and development, the delivery efficiency and safety of ADC drugs are expected to improve further, providing cancer patients with more precise and effective treatment options.
Indications for ADC Drugs
Breast Cancer: For patients with HER2-positive breast cancer, multiple ADC drugs are available. For example, Genentech's Kadcyla (Trastuzumab Emtansine), the first approved ADC drug for breast cancer, has demonstrated efficacy in treating HER2-positive metastatic breast cancer. Additionally, Daiichi Sankyo/AstraZeneca's Enhertu (Trastuzumab Deruxtecan) has shown exceptional therapeutic efficacy not only in HER2-positive breast cancer but also achieved groundbreaking progress in patients with HER2-low expressing breast cancer.
Hematologic Malignancies: ADC drugs also have broad applications in hematologic malignancies. For instance, Pfizer’s Besponsa (Inotuzumab Ozogamicin) is approved for the treatment of CD22-positive acute lymphoblastic leukemia (ALL). Similarly, Seattle Genetics/Takeda’s Adcetris (Brentuximab Vedotin) is used for the treatment of Hodgkin lymphoma and anaplastic large cell lymphoma.
Solid Tumors: In the field of solid tumor treatment, ADC drugs also demonstrate significant potential. Astellas Pharma’s Padcev (Enfortumab Vedotin-ejfc) is approved for the treatment of locally advanced or metastatic urothelial carcinoma. Immunomedics' Trodelvy (Sacituzumab Govitecan-hziy) is indicated for metastatic triple-negative breast cancer and other solid tumors.
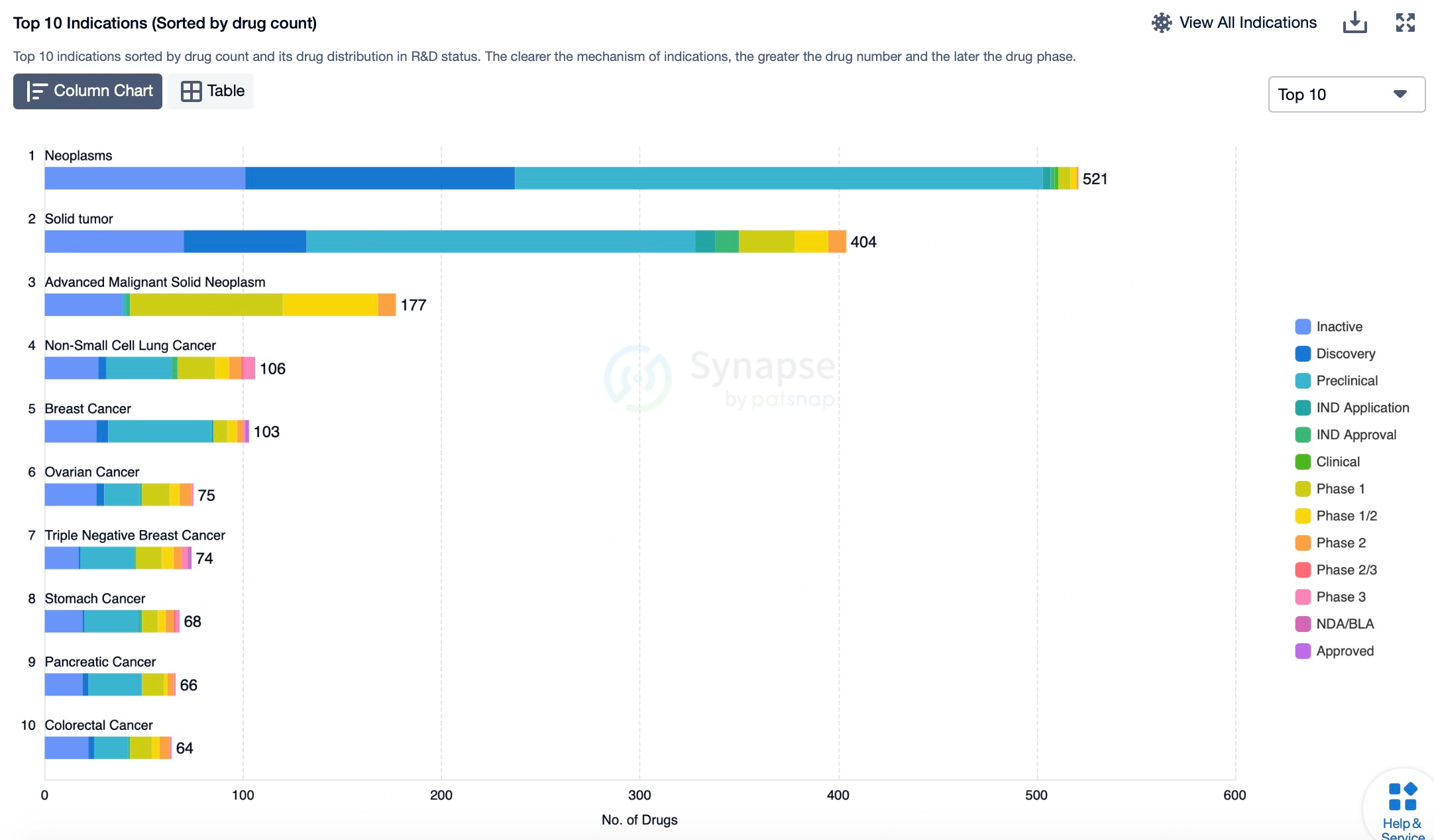
These ADC drugs employ a unique mechanism by combining highly specific monoclonal antibodies with potent cytotoxic agents to achieve targeted destruction of tumor cells, while minimizing damage to healthy tissues. As ADC technology continues to advance and clinical research progresses, it is anticipated that more ADC drugs targeting different indications will emerge in the future, providing patients with a broader range of personalized treatment options.
Regional Analysis of ADC Development
Globally, ADCs are experiencing robust growth, with North America and Europe leading the field due to their advanced technologies and mature regulatory frameworks. These regions benefit from abundant research and development resources, coupled with extensive expertise. Additionally, their stringent drug approval standards provide a solid foundation for the high-quality advancement of ADCs.
The North American market serves as the central hub for ADC research and development, boasting numerous leading biopharma companies at the forefront of technological innovation and clinical application of ADCs. Regulatory agencies in this region, such as the U.S. Food and Drug Administration (FDA), have accumulated substantial experience in evaluating and approving ADCs, paving the way for their expedited entry into the market. Similarly, the European market should not be overlooked. The European Medicines Agency (EMA) has set rigorous standards and guidelines to promote high-quality ADC development. Moreover, Europe’s strong foundation in biopharmaceuticals further strengthens its contribution to ADC advancements.
In Asia, particularly China, the ADC market is rapidly emerging as a key driving force in global ADC development. China’s vast patient population and rapidly growing demand for healthcare services create a significant market opportunity for ADCs. Additionally, the Chinese government has introduced a series of policies encouraging the development of innovative drugs. Coupled with the rise of domestic pharmaceutical companies and advancements in technology, China has made remarkable progress in ADC development.
With the ongoing transformation of the global ADC industry, more novel ADCs have been approved, increasing treatment choices and intensifying the competitive dynamics within the market. Intensified investment and R&D efforts from leading pharmaceutical companies indicate that ADC drugs will witness additional growth opportunities in the coming years.
Key Players in the ADC Development Market
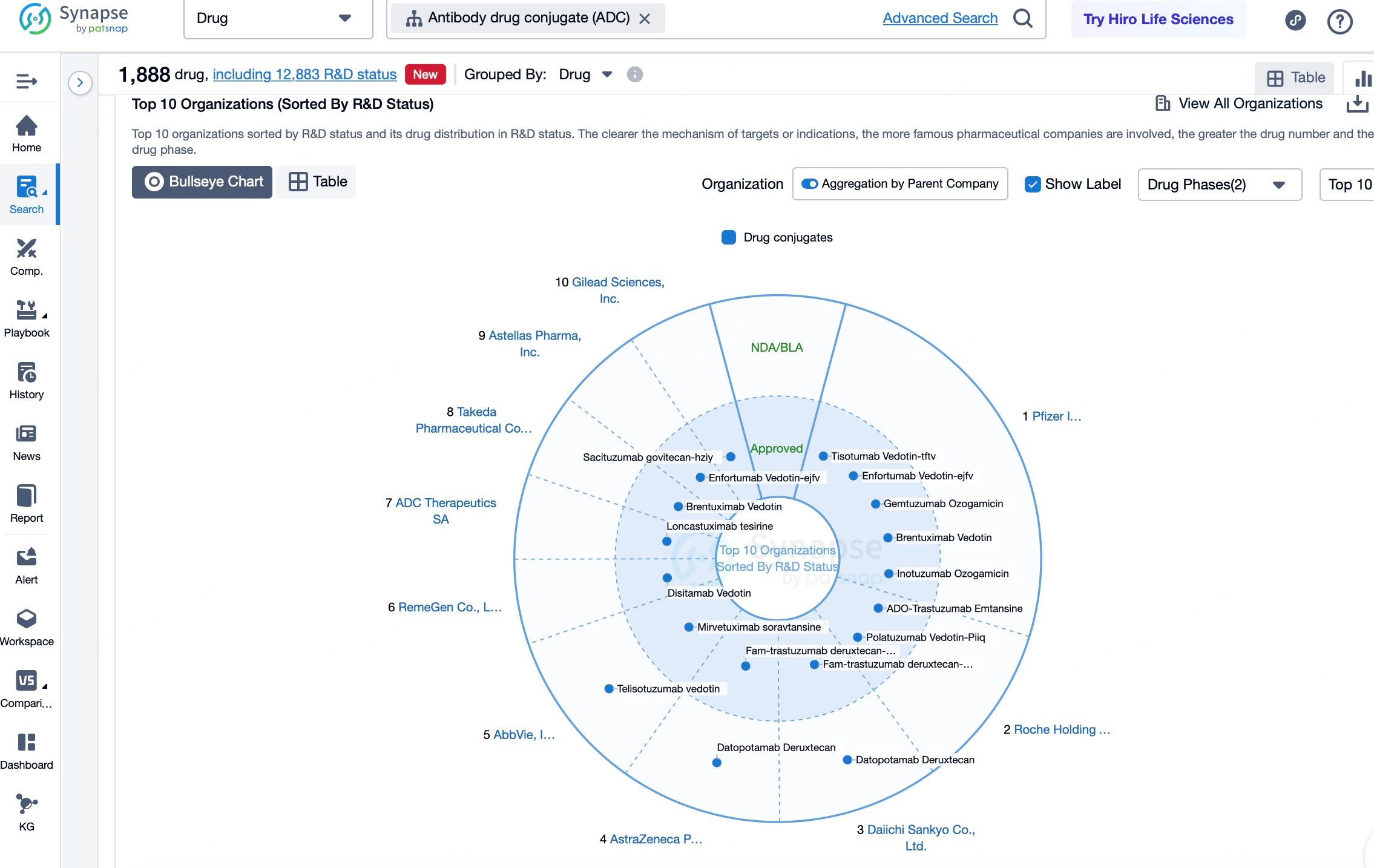
Seattle Genetics, now known as Seagen, is one of the pioneers in the ADC sector. As a leader in ADC development, Seagen possesses extensive research experience and has launched several marketed ADC products. Among its notable achievements, Adcetris (brentuximab vedotin) became the first approved treatment specifically for anaplastic large-cell lymphoma and the first new therapy in nearly 40 years to receive FDA approval as a frontline option for relapsed Hodgkin lymphoma. Seagen has also developed Padcev (enfortumab vedotin-ejfc) for the treatment of locally advanced or metastatic urothelial cancer. Beyond its clinical success, Seagen has expanded its influence through collaborations with various companies, cementing its position as a leader in the ADC field.
Genentech, a member of the Roche Group, holds a significant position in ADC development. The company has actively contributed to ADC innovation, focusing on treatments for various cancer indications. One of its flagship ADC products, Kadcyla (ado-trastuzumab emtansine), has been approved for patients with HER2-positive early breast cancer. Genentech’s sustained investment and innovation in the ADC field make it a formidable force driving advancements in ADC technology.
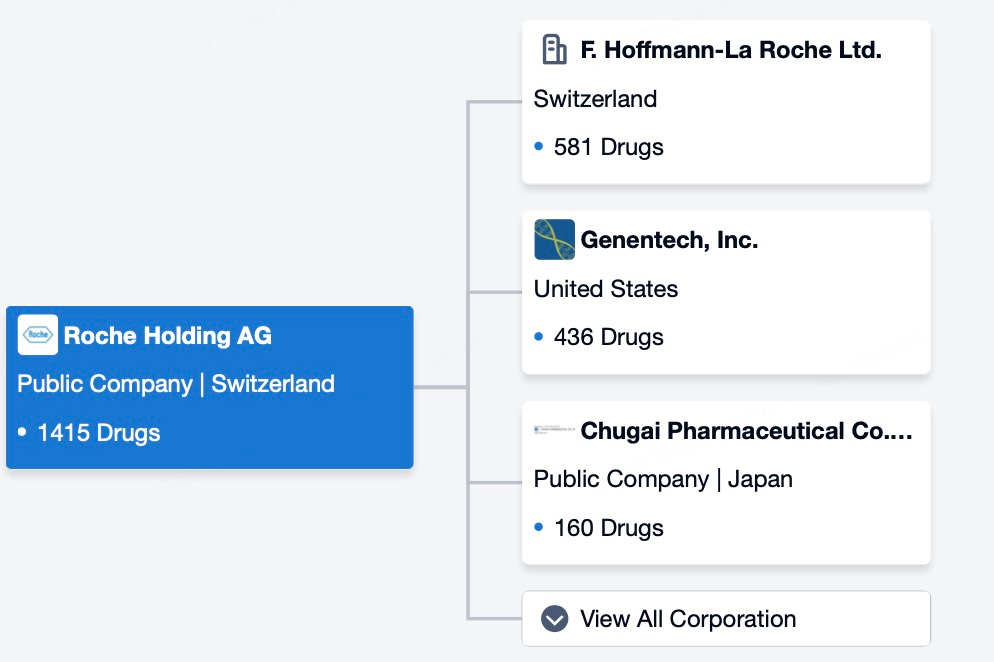
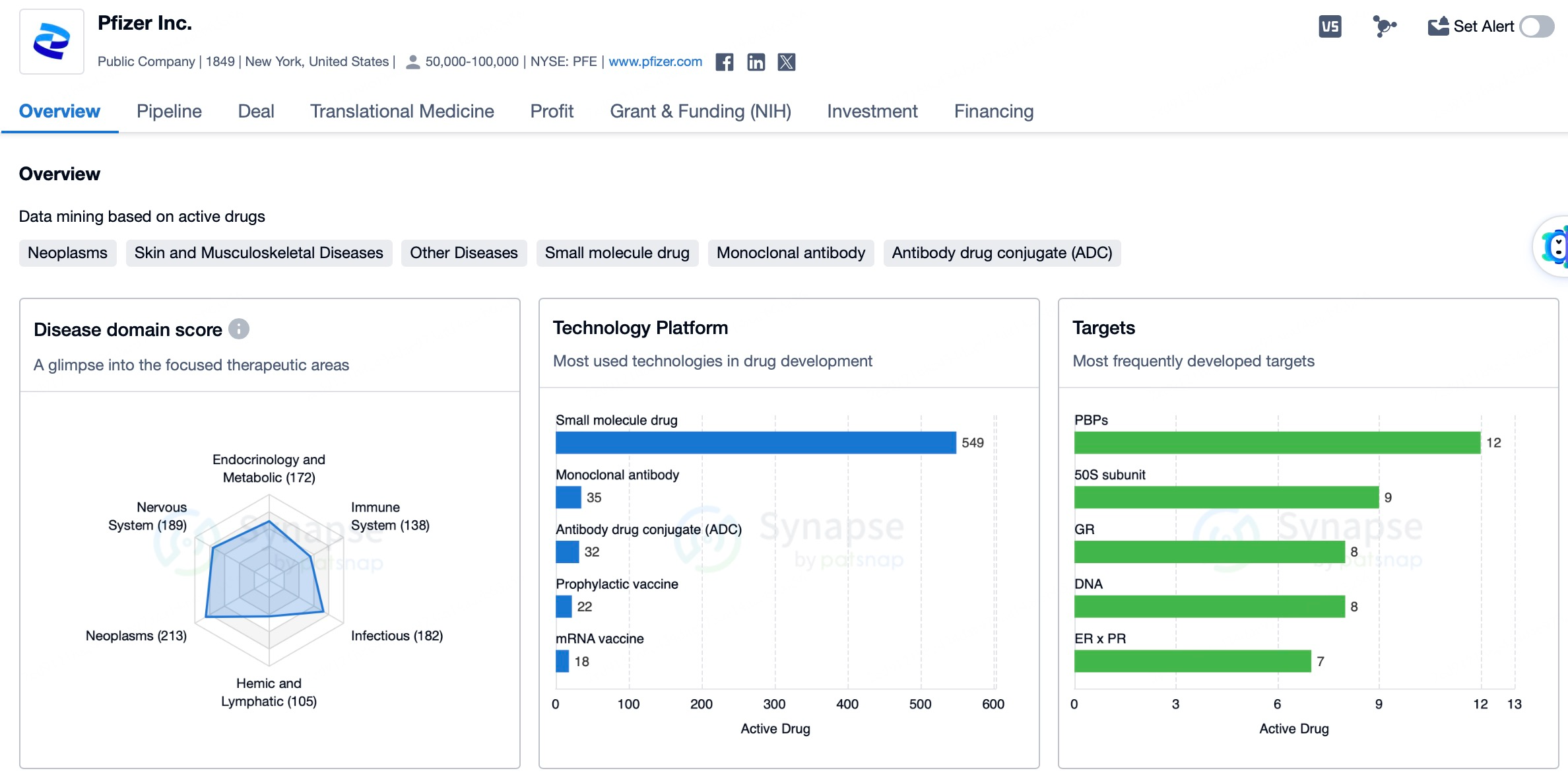
Pfizer possesses a diverse portfolio in the ADC space, dedicating itself to developing ADC therapies for hematologic malignancies. The company has launched several ADC products through partnerships and continues to expand its pipeline. For example, Pfizer’s Mylotarg (gemtuzumab ozogamicin) and Besponsa (inotuzumab ozogamicin) are used to treat acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), respectively. Notably, Pfizer further strengthened its leadership in the ADC sector by acquiring Seagen for $43 billion in 2023.
These companies play a significant role in the development of ADC drugs, not only driving advancements in ADC technology but also providing new treatment options for patients. With the continuous progress of ADC technology and the growing market demand, these companies will continue to play an essential role on a global scale.
Conclusion
ADCs demonstrate tremendous potential in precision medicine as an emerging therapeutic modality. By targeting specific antigens on cancer cells, ADCs can effectively deliver cytotoxic drugs, achieving selective tumor cell killing and offering novel treatment options for hard-to-treat cancers. In recent years, the successful market entry of various ADC drugs has not only highlighted the efficacy and safety of ADC therapies in clinical applications but also signaled the arrival of more innovative drugs, which will benefit a broader patient population.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Refrence
- 1.Dumontet, C., et al., Antibody-drug conjugates come of age in oncology. Nat Rev Drug Discov, 2023. 22(8): p. 641-661.
- 2.Bargh, J.D., et al., Cleavable linkers in antibody-drug conjugates. Chem Soc Rev, 2019. 48(16): p. 4361-4374.
- 3.Drago, J.Z., S. Modi, and S. Chandarlapaty, Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol, 2021. 18(6): p. 327-344.