The U.S. FDA grants Santhera approval for AGAMREE® (vamorolone) as a therapeutic for Duchenne Muscular Dystrophy
Santhera Pharmaceuticals has confirmed that the FDA has granted approval for AGAMREE® (vamorolone) oral suspension 40 mg/ml. This approval is specifically for treating Duchenne muscular dystrophy in patients who are aged 2 years and above.
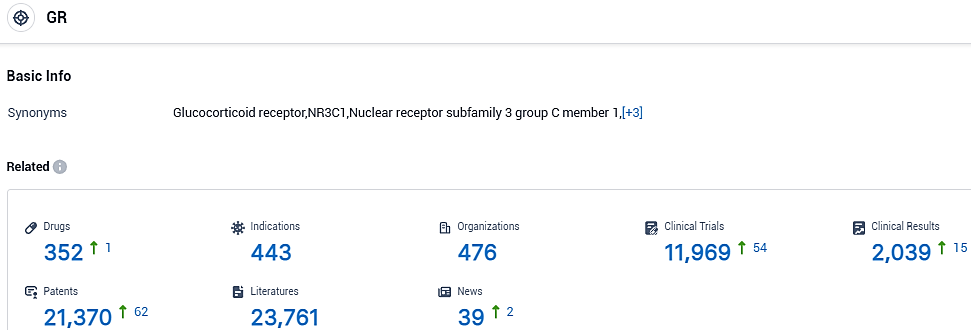
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"We are thrilled to have received FDA approval so quickly after the European Medicines Agency's CHMP expressed their positive stance," remarked Dario Eklund, Santhera's CEO. "This presents a significant turning point for DMD patients in dire need of an effective and tolerable treatment to fight this devastating ailment. Our collaboration with Catalyst Pharmaceuticals is set to continue as they gear up for the U.S. market launch of the product."
“The approval of this new medication provides Duchenne patients, and their families, added hope,” commented Pat Furlong, the founding president and CEO of Parent Project Muscular Dystroph. “Although steroids are the accepted treatment for Duchenne due to their beneficial role in delaying the disease’s progression, Vamorolone could offer an alternate steroid with a stronger tolerability profile, addressing a critical unmet health need for patients."
"Two thumbs up for the Duchenne community, who have been anticipating an alternative to the existing standard treatment for quite some time," said Eric Hoffman, the president and CEO of ReveraGen BioPharma. "It feels good to have worked with Santhera, the DMD patient community, researchers, and healthcare providers, towards achieving this critical landmark.”
The FDA’s endorsement of AGAMREE® was built upon data derived from the primary Phase 2b VISION-DMD study and corroborated with safety data from three open-label studies, inclusive of extension studies. These investigations in the development scheme were conducted by Santhera’s associate, ReveraGen, as well as 32 academic clinical trial facilities dispersed across 11 nations.
In view of the FDA’s sanctioning of AGAMREE for DMD, Catalyst is due to disburse USD 36 million to Santhera. This accounts for a USD 10 million authorization milestone for the Company, plus an additional USD 26 million to satisfy contracted third-party milestone liabilities.
Moreover, according to the contract terms, Catalyst is set to transfer sales-based milestones amounting to up to USD 105 million to Santhera along with low-teen percentage royalties, while taking on Santhera's related third-party royalty obligations on vamorolone revenues in all indications within North America.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 31, 2023, there are 352 investigational drugs for the GR target, including 443 indications, 476 R&D institutions involved, with related clinical trials reaching 11969,and as many as 21370 patents.
Santhera prepares to hand over the US marketing rights for AGAMREE to its partner, Catalyst Pharmaceuticals, planned to release the product in the US in Q1-2024. Catalyst Pharmaceuticals holds the exclusive license for AGAMREE in North America. Meanwhile, in Europe, after receiving a favorable opinion from the CHMP on October 12, 2023, the European Commission is anticipated to authorize AGAMREE (vamorolone) for DMD treatment by the end of 2023.