Unleashing the Power of Clocortolone Pivalate: A Comprehensive Review on R&D Breakthroughs
Clocortolone Pivalate's R&D Progress
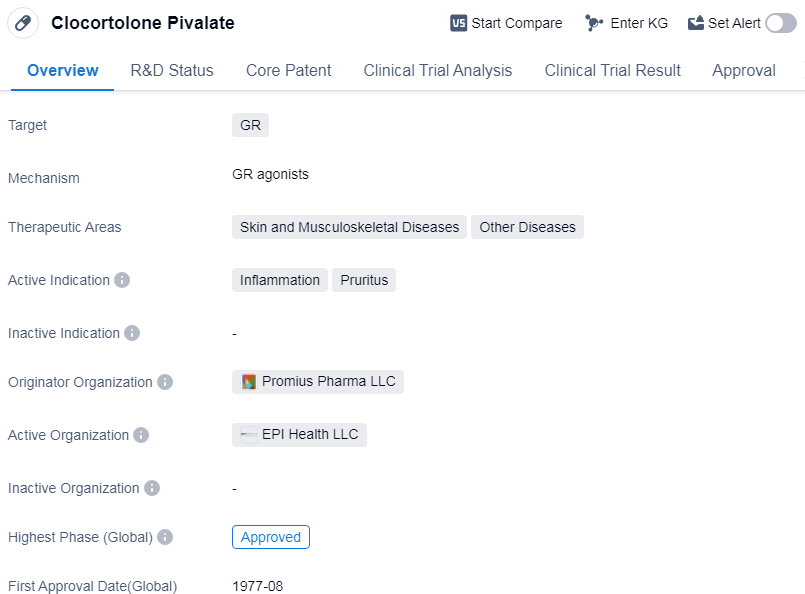
Clocortolone Pivalate is a small molecule drug that targets the glucocorticoid receptor (GR). It is primarily used in the treatment of skin and musculoskeletal diseases, as well as other diseases. The drug is specifically indicated for the management of inflammation and pruritus.
The originator organization of Clocortolone Pivalate is Promius Pharma LLC, which played a crucial role in the development and approval of the drug. Clocortolone Pivalate has reached the highest phase of development, which is the approved stage. This indicates that the drug has successfully undergone rigorous testing and evaluation, demonstrating its safety and efficacy.The first approval of Clocortolone Pivalate took place in August 1977 in the United States.
Clocortolone Pivalate's primary therapeutic areas are skin and musculoskeletal diseases, which suggests that it is particularly effective in treating conditions related to these areas. Additionally, it is also indicated for other diseases, although specific details about these diseases are not provided.
The drug's active indications are inflammation and pruritus. Inflammation refers to the body's response to injury or infection, characterized by redness, swelling, and pain. Pruritus, on the other hand, refers to itching, which can be caused by various factors such as allergies, skin conditions, or systemic diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Clocortolone Pivalate: GR agonists
From a biomedical perspective, GR agonists refer to substances or drugs that activate the glucocorticoid receptor (GR). The GR is a type of nuclear receptor found in cells, particularly in the cytoplasm. When a GR agonist binds to the GR, it triggers a series of molecular events that ultimately lead to changes in gene expression. These changes can have various effects on the body, including anti-inflammatory and immunosuppressive actions. GR agonists are commonly used in the treatment of conditions such as asthma, autoimmune diseases, and certain types of cancer. They can help reduce inflammation, suppress the immune system, and regulate various physiological processes.
Drug Target R&D Trends for Clocortolone Pivalate
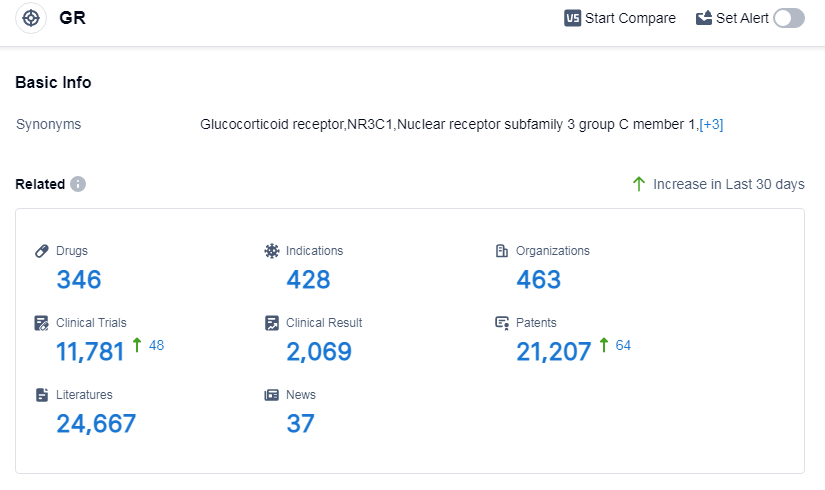
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 346 GR drugs worldwide, from 463 organizations, covering 428 indications, and conducting 11781 clinical trials.
The analysis of the target GR in the pharmaceutical industry reveals a competitive landscape with several key players. Novartis AG, Shionogi & Co., Ltd., GSK Plc, Bausch Health Cos., Inc., and Pfizer Inc. are leading companies with a strong focus on R&D and a significant number of approved drugs. The top indications include eczema, asthma, psoriasis, and skin diseases.
Small molecule drugs dominate the drug type analysis, but other innovative drug types such as ADCs, monoclonal antibodies, and antisense oligonucleotides show potential for future development. The United States and China are leading in terms of approved drugs, with China showing significant progress and emerging as a major player in the target GR field.
Overall, the target GR presents a competitive landscape with opportunities for growth and innovation.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Clocortolone Pivalate is a small molecule drug that targets the glucocorticoid receptor and is primarily used in the treatment of skin and musculoskeletal diseases. It has been approved since 1977 and is indicated for managing inflammation and pruritus. The drug's originator organization is Promius Pharma LLC, and its first approval occurred in the United States.