Is Bexagliflozin approved by the FDA?
The FDA approved Bexagliflozin under the brand name Brenzavvy on January 20, 2023. This approval provides a new option for managing blood sugar levels in adults with type 2 diabetes, complementing diet and exercise.
Uses for Bexagliflozin
Bexagliflozin is primarily used to improve blood sugar control in adults with type 2 diabetes mellitus. It is not intended for use in individuals with type 1 diabetes. The medication may also be prescribed for other purposes not listed in the official medication guide.
Administration and Dosage
Bexagliflozin is taken as an oral tablet, typically dosed at 20 mg once daily in the morning, with or without food. The tablet should be swallowed whole, without crushing or chewing. Treatment may also involve dietary changes, exercise, weight control, and regular medical checkups to monitor blood sugar levels.
Precautions and Side Effects
Warnings:
- Do not use Bexagliflozin if you have severe kidney disease or diabetic ketoacidosis.
- Bexagliflozin may increase the risk of lower leg amputation, especially in individuals with prior amputation, foot ulcers, heart disease, circulation problems, or nerve damage.
- Dehydration is a potential risk while taking Bexagliflozin, which can lead to severely low blood pressure or serious electrolyte imbalance.
- Serious infections in the genital area can occur. Seek medical help if symptoms such as burning, itching, odor, discharge, pain, or swelling in the genital or rectal area occur.
Common Side Effects:
- Increased or urgent need to urinate, especially at night
- Pain and burning during urination
- Vaginal itching or discharge
Serious Side Effects:
- Nausea, vomiting, stomach pain
- Tiredness or trouble breathing
- Signs of bladder infection, such as pain or burning during urination, increased urination, blood in the urine, fever, or pelvic pain
- Symptoms of low blood sugar, including headache, weakness, drowsiness, confusion, hunger, sweating, irritability, dizziness, fast heart rate, and feeling anxious or shaky
- Fever, fatigue, redness, swelling, pain, or tenderness in the genital or anal area
Before Taking Bexagliflozin:
- Avoid using Bexagliflozin if allergic or if you have severe kidney disease.
- Discuss your medical history with your doctor, including any history of type 1 diabetes, diabetic ketoacidosis, low blood pressure, bladder infections, foot ulcers, circulatory problems, liver or kidney disease, frequent alcohol consumption, or planned surgeries.
- Inform your doctor if you are pregnant, planning to become pregnant, or breastfeeding.
- It is not recommended to use Bexagliflozin during the second or third trimester of pregnancy.
Interactions with Other Drugs
Bexagliflozin can interact with various medications, including other diabetes medications. Inform your doctor about all the medications you are currently taking, including prescription, over-the-counter drugs, vitamins, and herbal supplements.
Conclusion
Bexagliflozin (Brenzavvy) was approved by the FDA on January 20, 2023, for improving blood sugar control in adults with type 2 diabetes mellitus. This medication, part of the SGLT-2 inhibitors class, provides a valuable treatment option for managing diabetes when used alongside diet and exercise. Patients should be aware of the potential side effects and necessary precautions while using Bexagliflozin and consult their healthcare provider for personalized advice and monitoring.
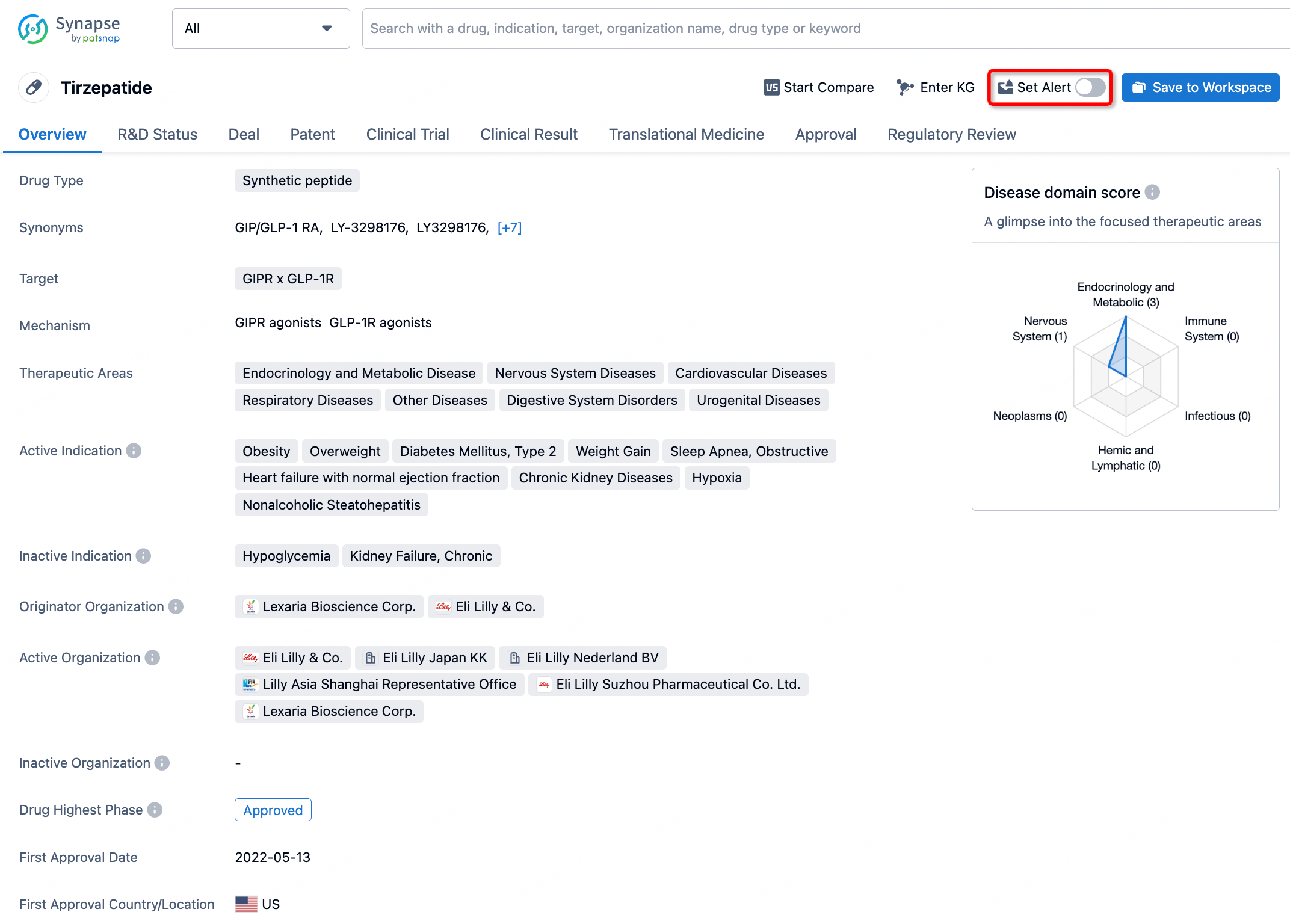
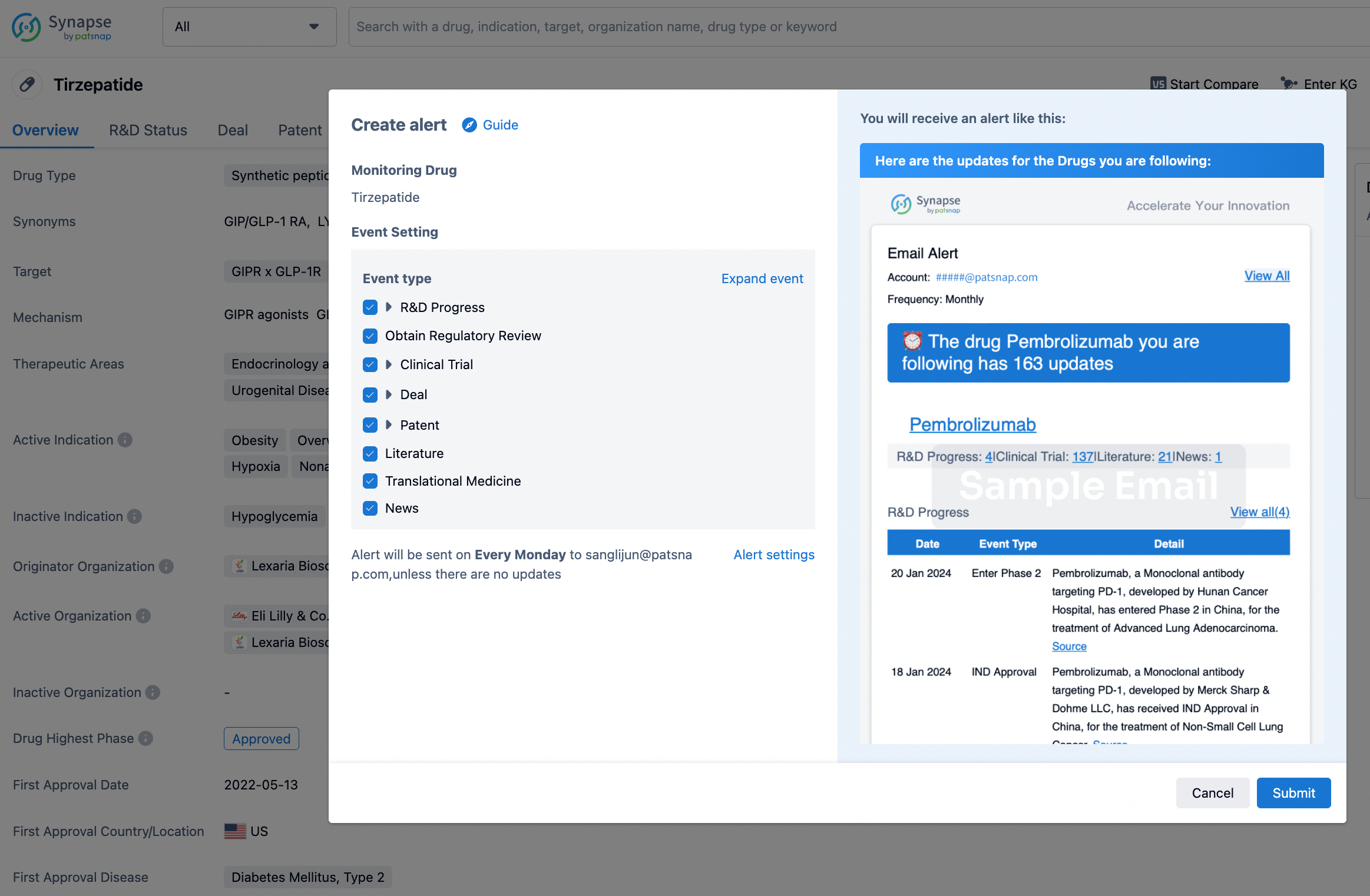
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!