Exploring TILT-123's R&D successes and its clinical results at the 2023 SITC
The clinical result of T-cell inducing oncolytic virus (igrelimogene litadenorepvec; TILT-123) shows safety, anti-tumor activity and induction of immune responses in advanced solid tumor patients was reported in 2023 SITC Congress, offering significant insights into its potential therapeutic benefits.
TILT-123's R&D Progress
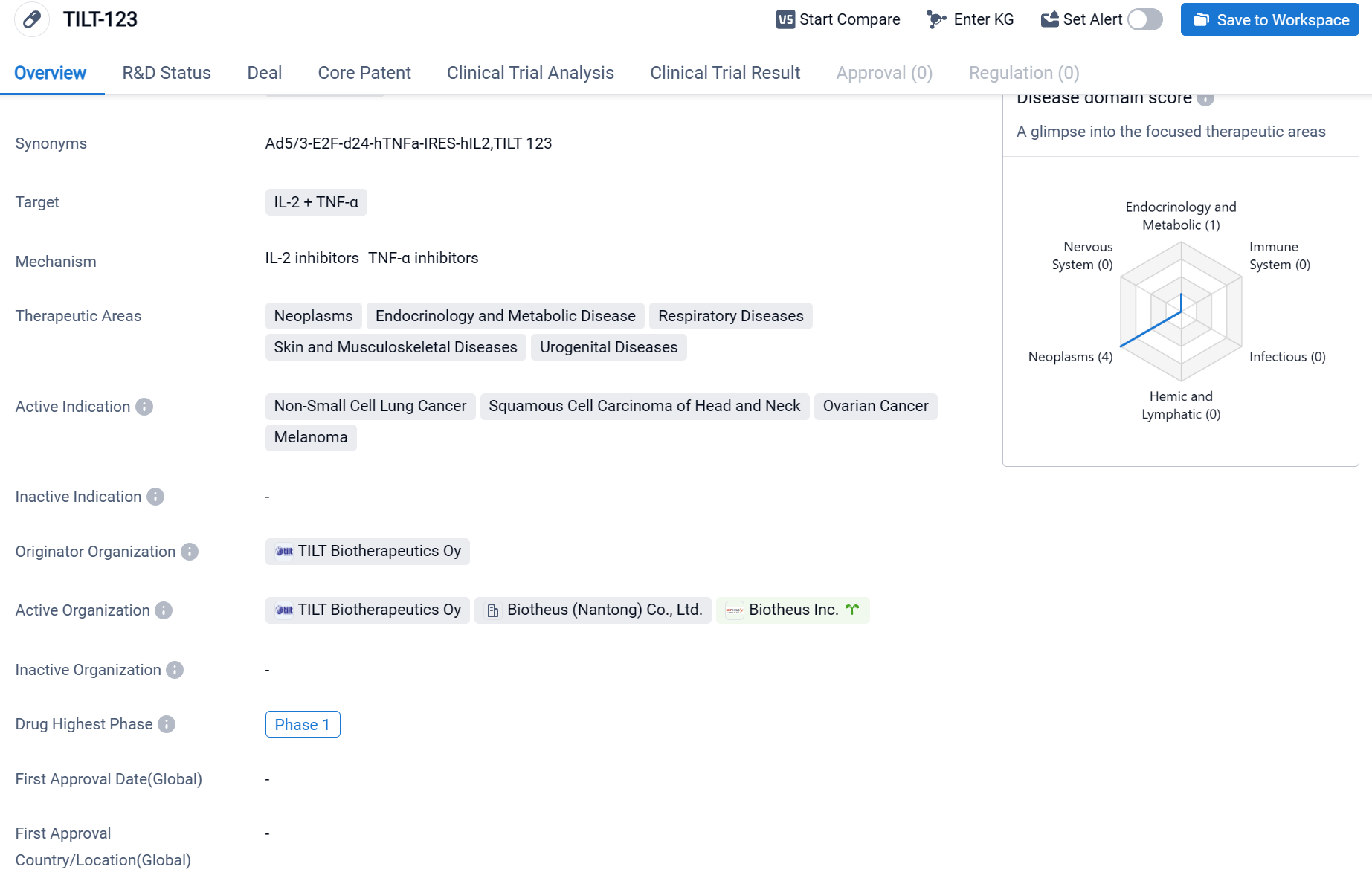
TILT-123 is an oncolytic virus drug that targets IL-2 and TNF-α. It is being developed by TILT Biotherapeutics Oy, an organization specializing in biomedicine. The therapeutic areas that TILT-123 aims to address include neoplasms, endocrinology and metabolic disease, respiratory diseases, skin and musculoskeletal diseases, and urogenital diseases. This suggests that the drug has the potential to be used in the treatment of a wide range of medical conditions.
According to the Patsnap Synapse, TILT-123 s currently in Phase 1, which is the highest phase of development globally. And the clinical trial areas for TILT-123 are primarily in the United States, France and Denmark. The key indication is Melanoma. 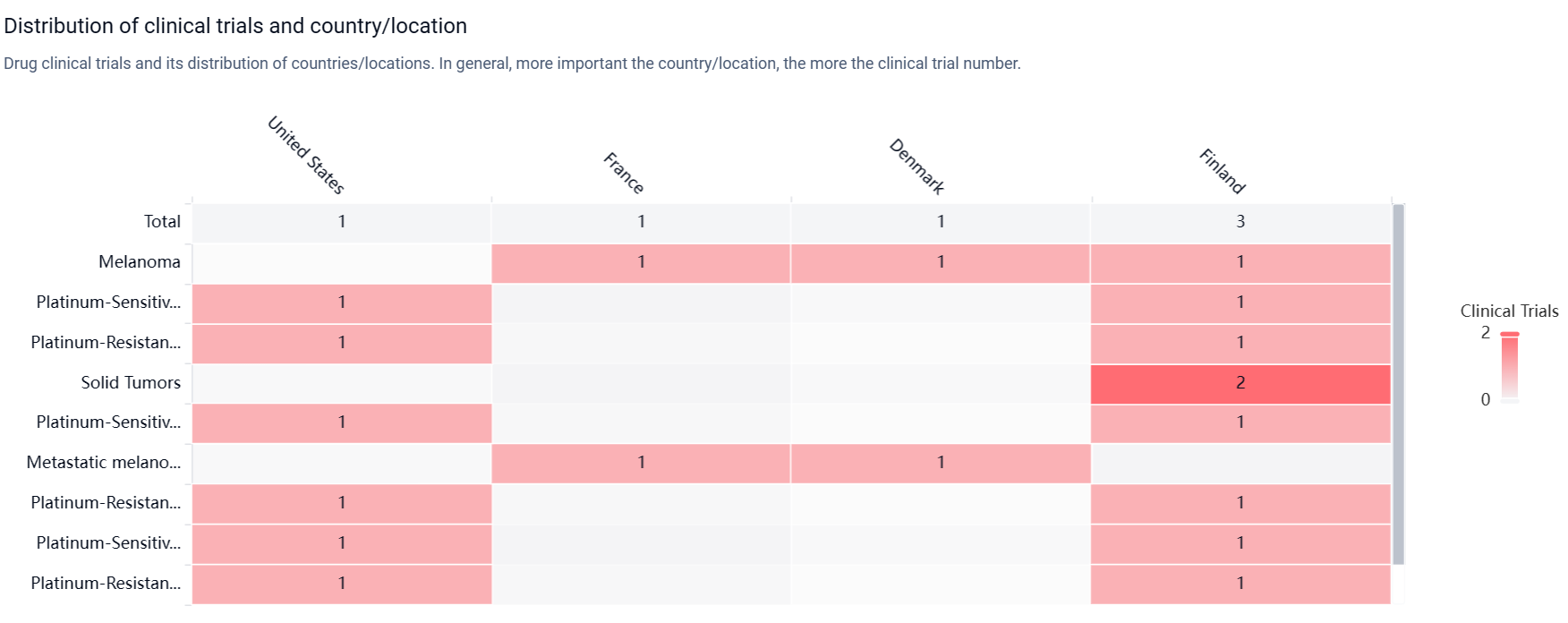
Detailed Clinical Result of TILT-123
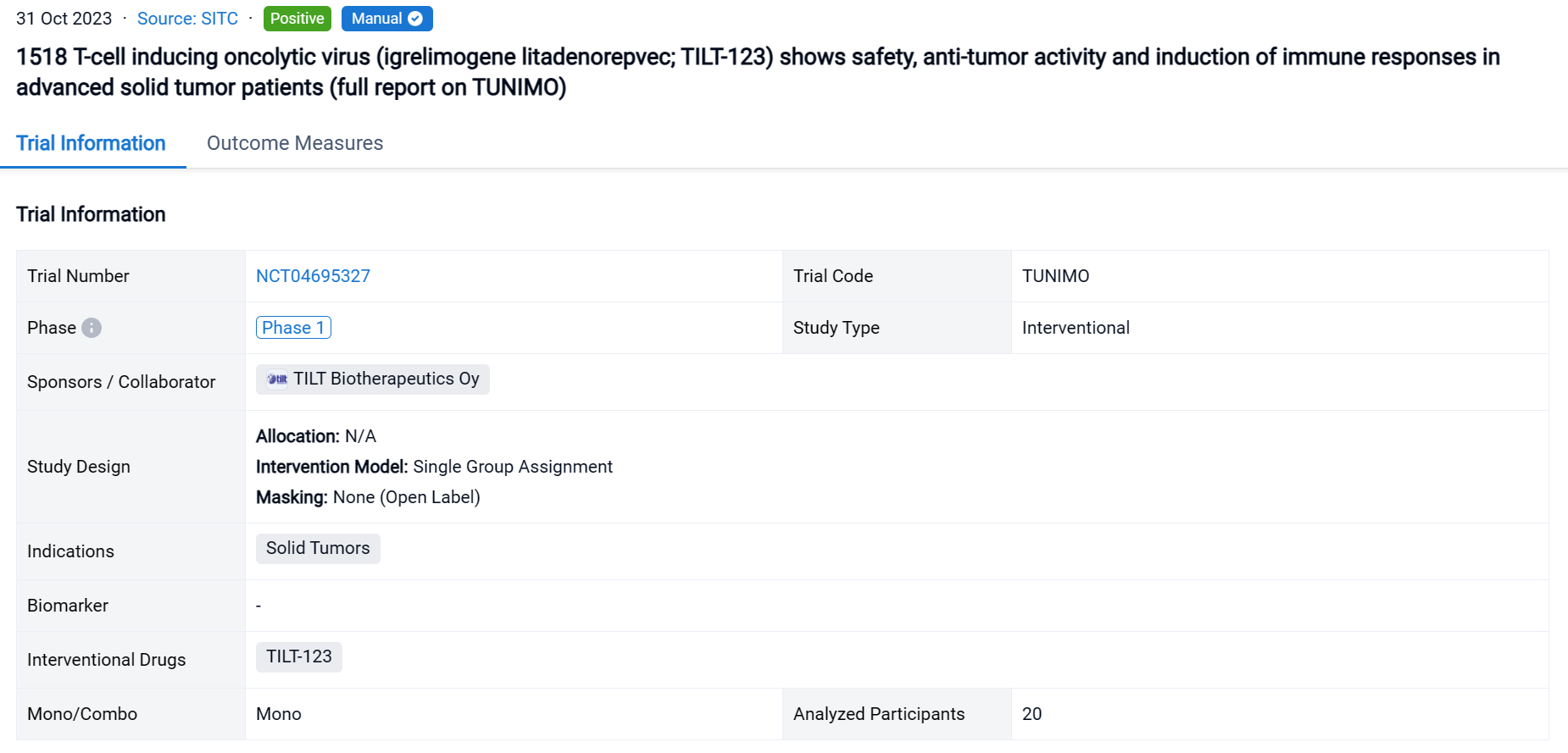
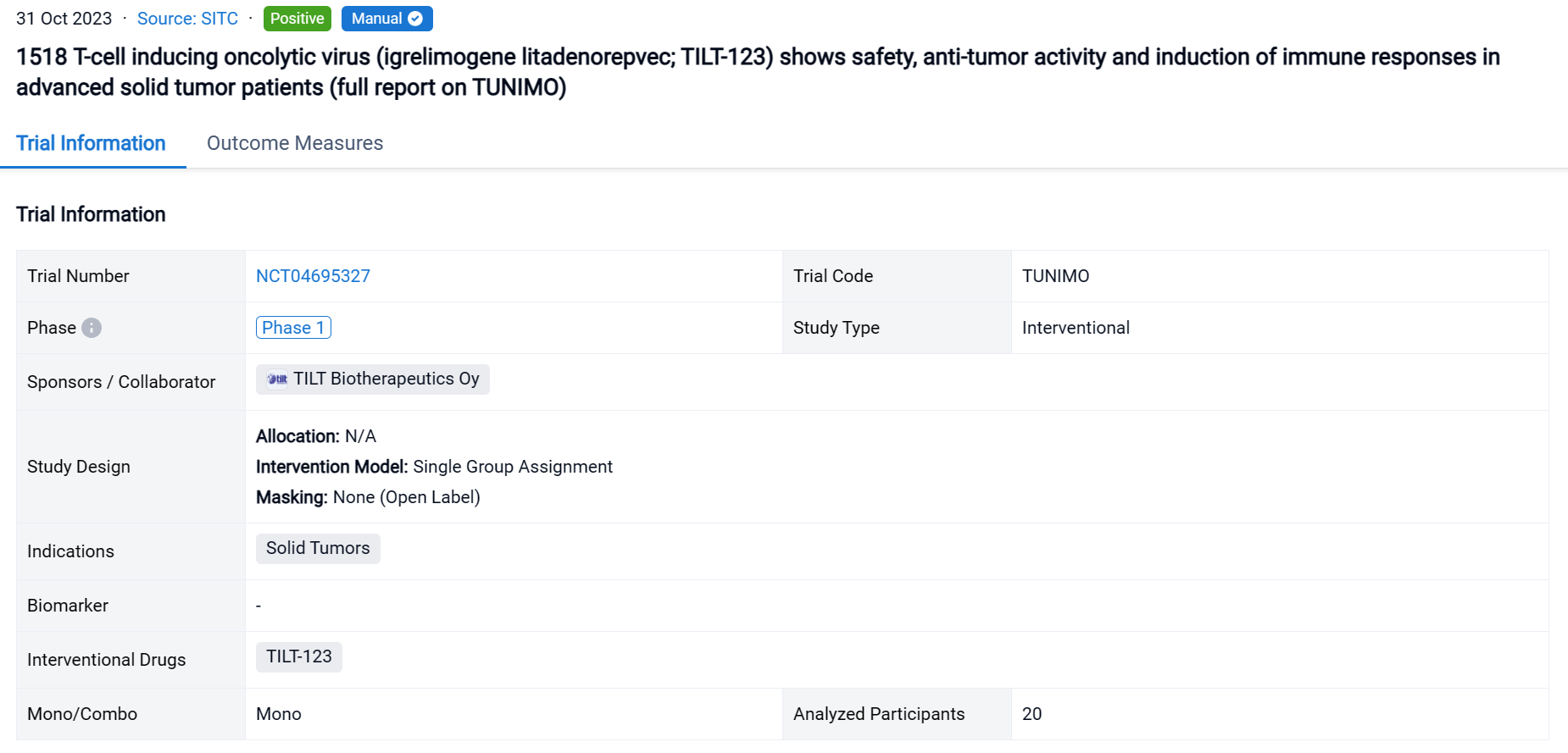
TUNIMO (NCT04695327) is a single-armed open-label phase 1 clinical trial designed to assess the safety of TILT-123 monotherapy in patients with advanced solid tumors which are refractory to standard therapy.
In this study, the trial followed a 3+3 dose-escalation design where patients received a dose of TILT-123 intravenously, followed by at least five planned intratumoral and/or intravenous injections. The primary endpoint was safety by day 85, assessed by adverse events, vital signs, laboratory values and electrocardiogram. Secondary endpoints included evaluation of tumor responses, neutralizing antibodies, analysis of biopsies, virus persistence in blood and shedding. Enrollment was completed on Aug 31st 2023.
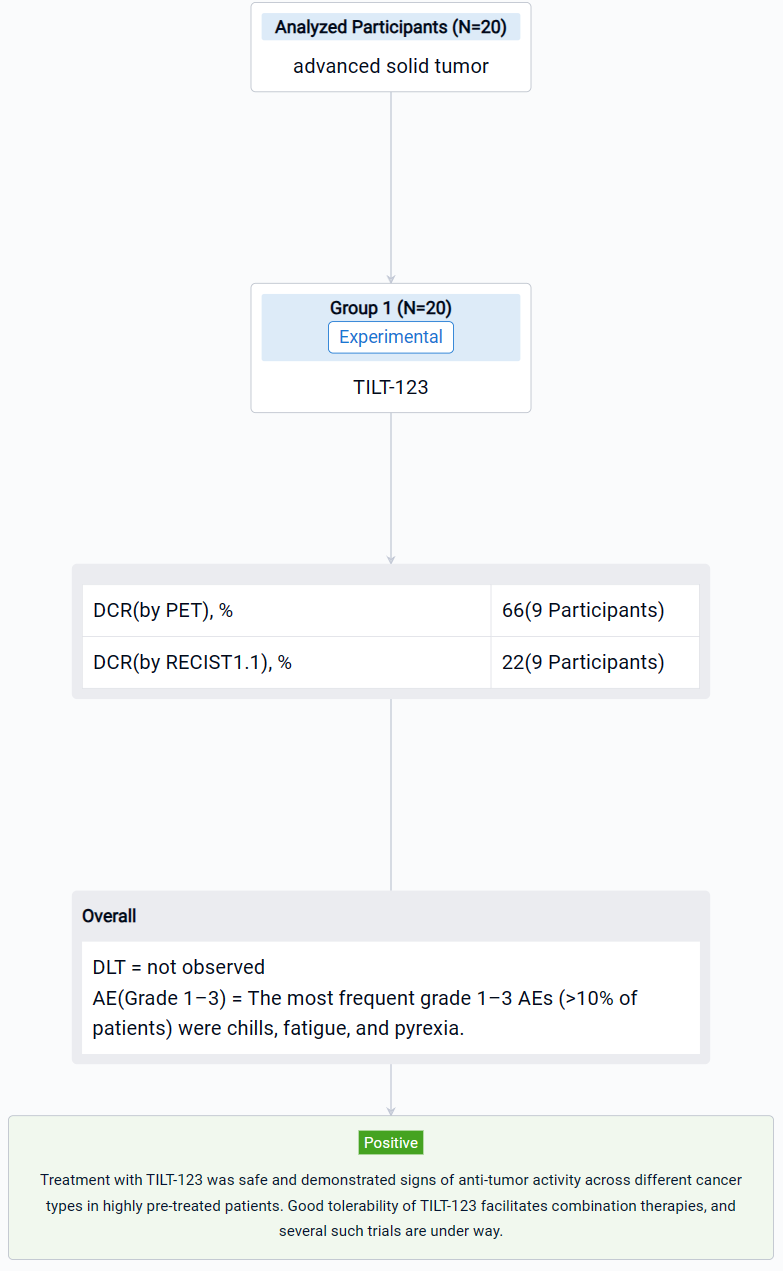
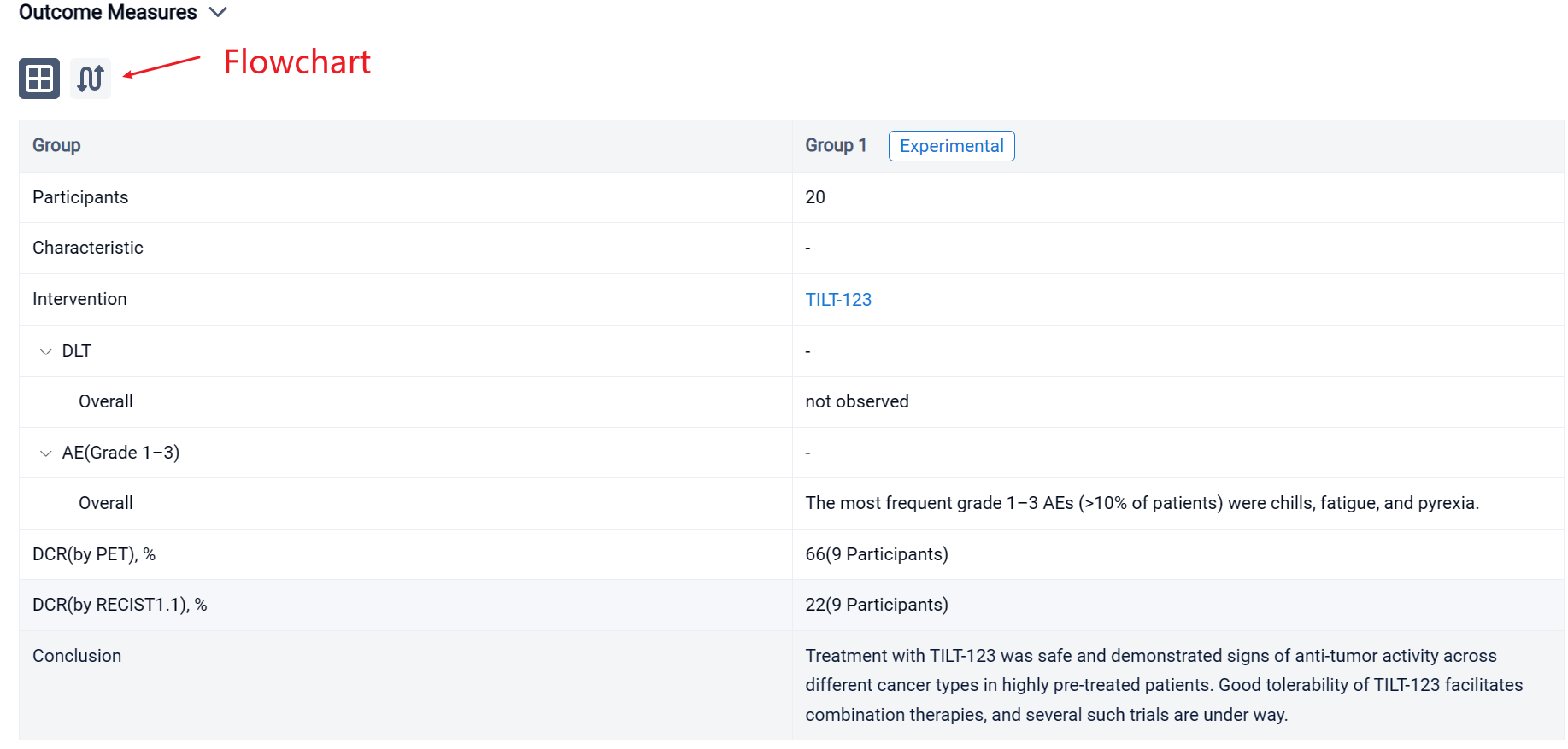
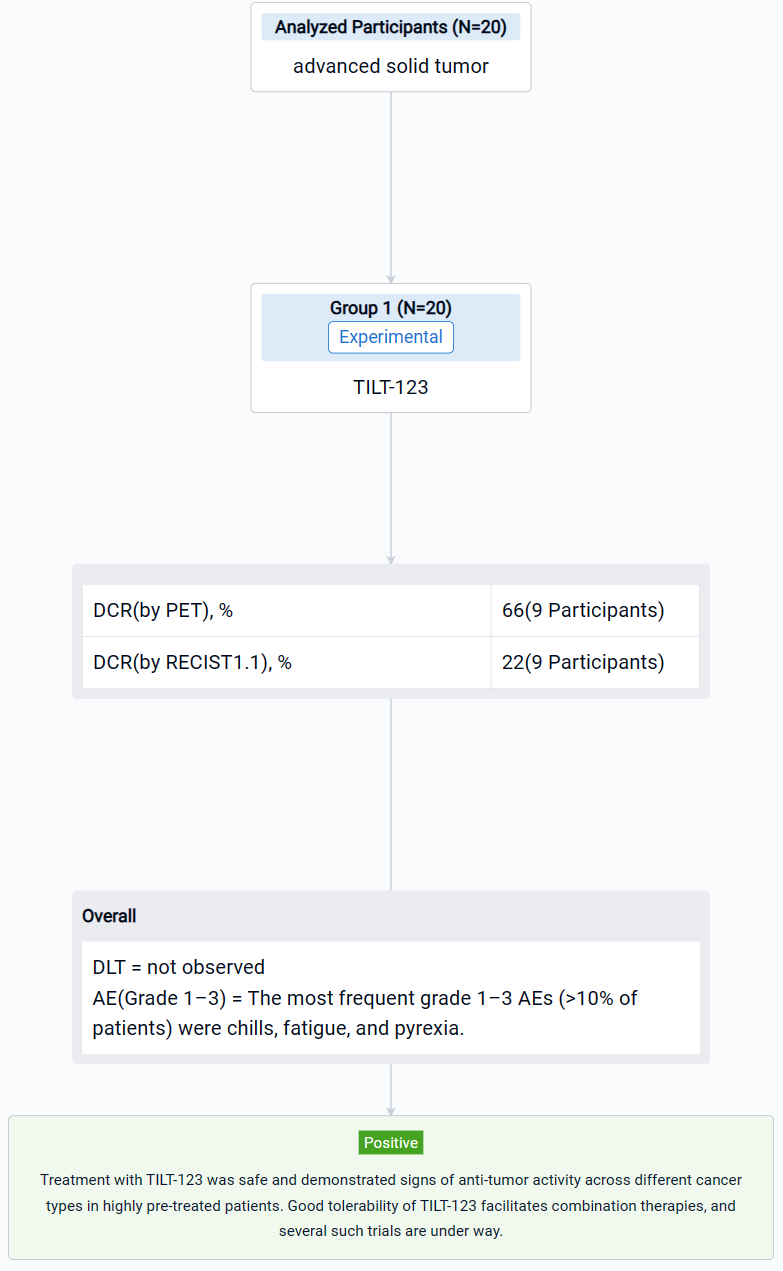
The result showed that 20 patients were enrolled, with a median age of 58 (33–72) years. The most prevalent cancer types were sarcomas (35%), melanoma (15%) and ovarian cancer (10%). Patients were heavily pretreated, receiving a median of 4.5 (range 0–16) lines of systemic therapy prior to trial entry. Patients received at least 3 doses of TILT-123 and dose-limiting toxicities were not observed. The most frequent grade 1–3 AEs (>10% of patients) were chills, fatigue, and pyrexia. By Aug 31st, 9 patients had been evaluated on d78 for response with RECIST 1.1, iRECIST or PET-based criteria. The overall disease control rate by PET was 6/9 (66%), including 4/9 (44%) stable metabolic disease and 2/9 (22%) minor or partial metabolic response. 2/9 (22%) patients showed disease control by RECIST1.1, including 1/9 (11%) partial response. Virus was detected in biopsies of injected and non-injected lesions. High virus amounts were seen in blood at one hour, with lower levels at 16 hours, and virus could still be detected several weeks later. Shedding into urine/saliva was negligible. Most patients had undetectable baseline neutralizing antibodies towards the chimeric 5/3 capsid, but titers increased after treatment. Biological data supporting T-cell infiltration into the tumor, was generated. Analysis of the serum proteome and cellular compartments revealed systemic immune activation following intravenous and intratumoral delivery.
It can be concluded that treatment with TILT-123 was safe and demonstrated signs of anti-tumor activity across different cancer types in highly pre-treated patients. Good tolerability of TILT-123 facilitates combination therapies, and several such trials are under way (NCT04217473, NCT05271318, NCT05222932).
How to Easily View the Clinical Results Using Synapse Database?
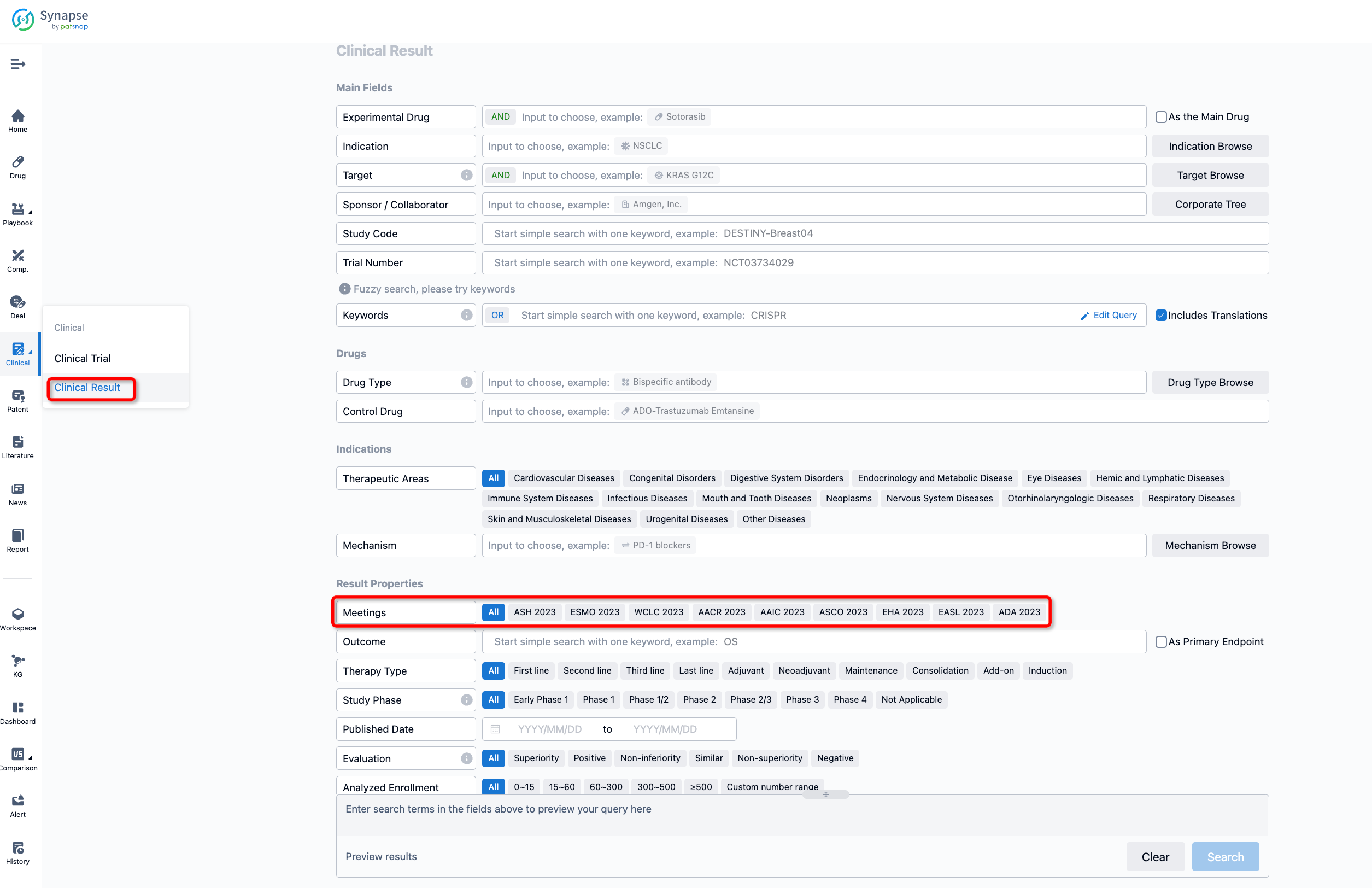
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
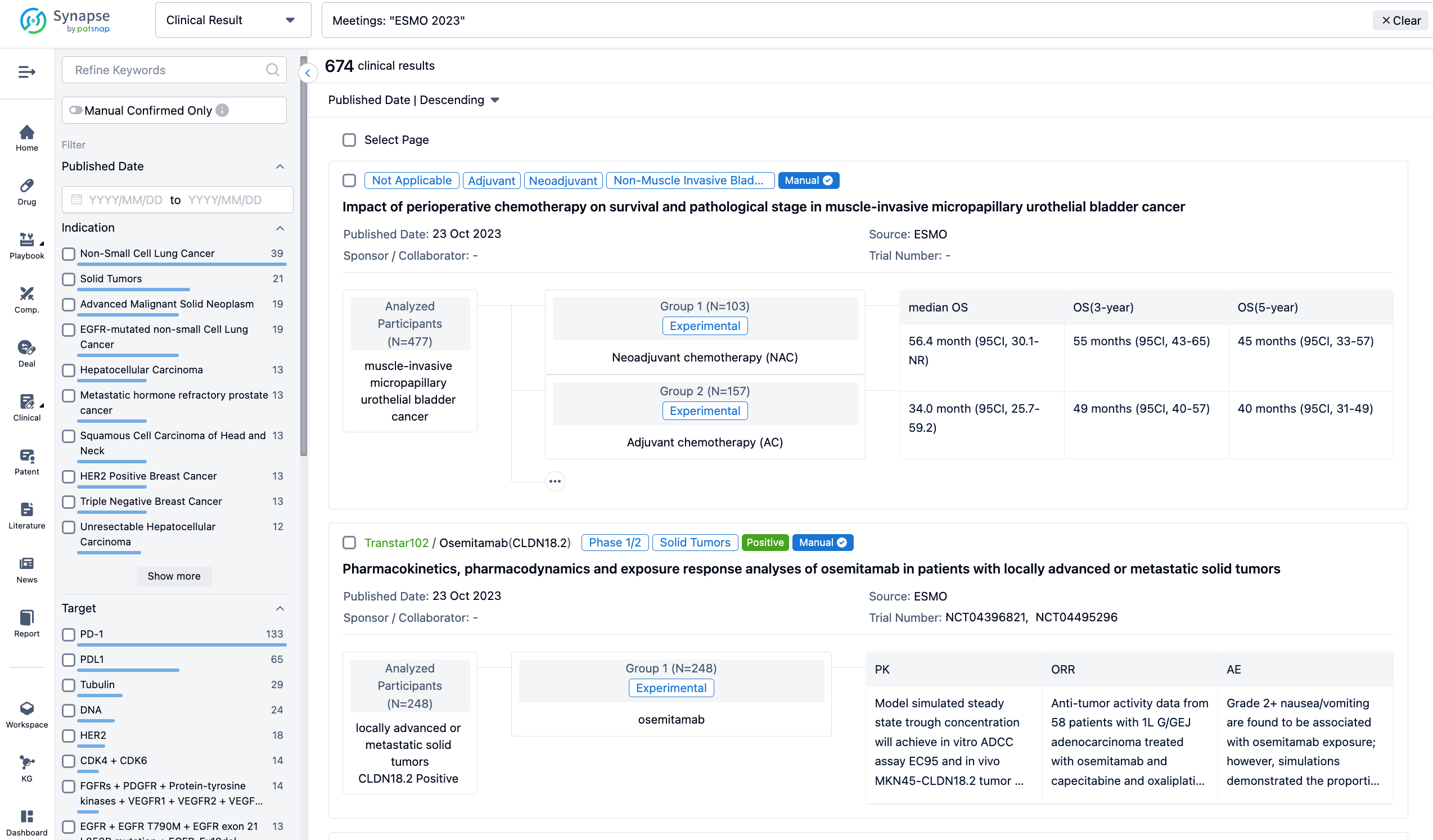
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
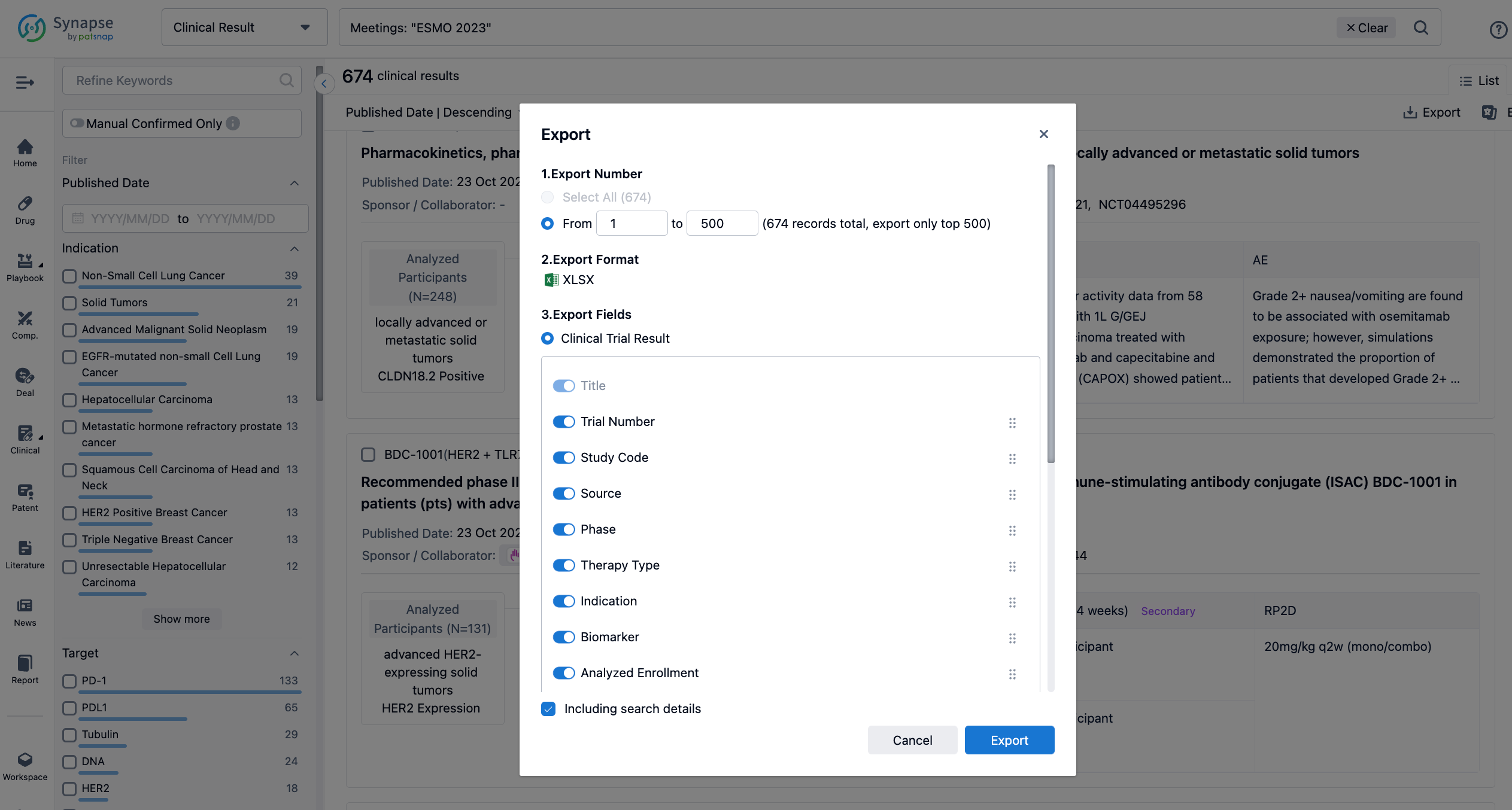
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!