Pharmaceutical Insights: Cocaine Hydrochloride's R&D Progress
Cocaine Hydrochloride's R&D Progress
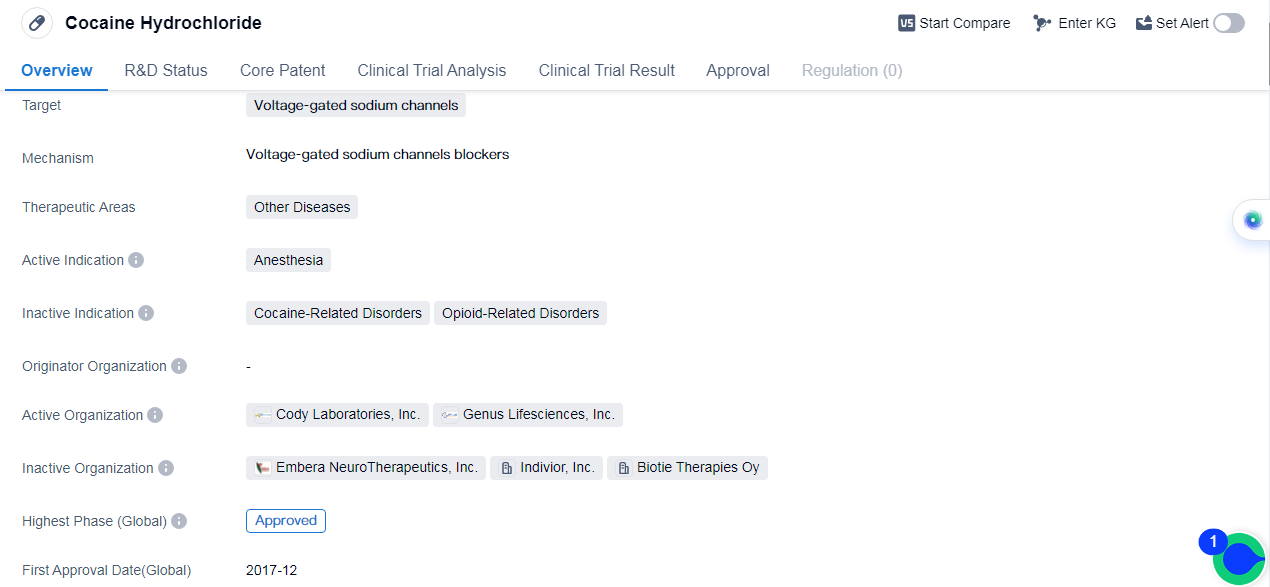
Cocaine Hydrochloride is a small molecule drug that targets voltage-gated sodium channels. It is primarily used in the field of biomedicine for anesthesia purposes. The drug has been approved in the global markets, with its highest phase being approved.
The first approval of Cocaine Hydrochloride took place in December 2017 in the United States. This indicates that the drug has successfully undergone the necessary clinical trials and regulatory processes to be deemed safe and effective for its intended use.
As a small molecule drug, Cocaine Hydrochloride is designed to interact with voltage-gated sodium channels. These channels play a crucial role in the transmission of electrical signals in nerve cells. By targeting these channels, the drug can modulate the activity of the nervous system, leading to its anesthetic effects.
While the therapeutic areas of Cocaine Hydrochloride are categorized as "Other Diseases," it is important to note that its primary indication is anesthesia. This suggests that the drug is primarily used to induce a loss of sensation or consciousness during medical procedures.
The approval of Cocaine Hydrochloride in the global markets highlights its recognized safety and efficacy profiles. This indicates that the drug has met the necessary regulatory standards and has undergone rigorous testing to ensure its suitability for use.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Cocaine Hydrochloride: Voltage-gated Sodium Channels Blockers
Voltage-gated sodium channels blockers are a class of drugs that specifically target and inhibit the function of voltage-gated sodium channels. These channels are integral membrane proteins found in the cell membranes of excitable cells, such as neurons and muscle cells. They play a crucial role in the generation and propagation of action potentials, which are electrical signals involved in nerve transmission and muscle contraction.
By blocking voltage-gated sodium channels, these drugs prevent the influx of sodium ions into the cells, thereby inhibiting the rapid depolarization phase of the action potential. This effectively reduces or prevents the generation of electrical signals, leading to the suppression of nerve activity or muscle contraction.
Voltage-gated sodium channel blockers are commonly used in the field of biomedicine to treat various conditions related to abnormal electrical activity, such as epilepsy, cardiac arrhythmias, and neuropathic pain. By selectively targeting specific subtypes of voltage-gated sodium channels, these drugs can provide therapeutic benefits while minimizing unwanted side effects.
It is important to note that the specific mechanism of action and efficacy of voltage-gated sodium channel blockers may vary depending on the particular drug and its target subtype of sodium channels. Therefore, the use of these blockers should be carefully considered and prescribed by healthcare professionals based on the individual patient's condition and medical history.
Drug Target R&D Trends for Cocaine Hydrochloride
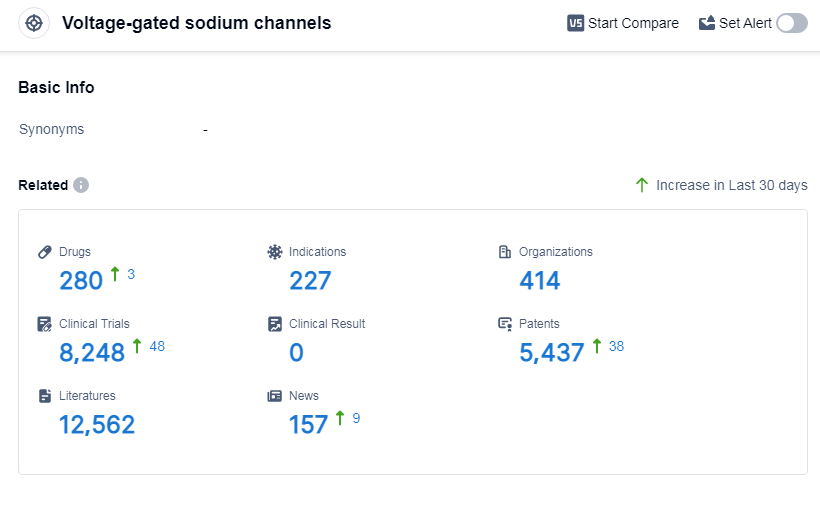
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 280 Voltage-gated sodium channels drugs worldwide, from 414 organizations, covering 227 indications, and conducting 8248 clinical trials.
The analysis of the target voltage-gated sodium channels reveals a competitive landscape with several companies actively involved in drug development. Novartis AG, Pfizer Inc., Johnson & Johnson, Sumitomo Chemical Co., Ltd., and Jiangsu Hengrui Pharmaceutical Group Co. Ltd. are the companies with the highest number of drugs in various development phases. The most common indications for approved drugs include anesthesia, arrhythmias, cardiac, seizures, and pain. Small molecule drugs dominate the development landscape, followed by antisense oligonucleotides and adeno-associated virus-based gene therapy. China, the United States, and Japan are leading in terms of drug development, with China showing significant progress. Overall, the target voltage-gated sodium channels present a promising area for future development and innovation in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Cocaine Hydrochloride is a small molecule drug that targets voltage-gated sodium channels. It has been approved for use in anesthesia and has undergone the necessary clinical trials and regulatory processes. The drug's approval in the global markets further emphasizes its recognized safety and efficacy.