Unleashing the Power of Eletriptan hydrobromide: A Comprehensive Review on R&D Breakthroughs, Action Mechanisms, and Drug Target
Eletriptan hydrobromide's R&D Progress
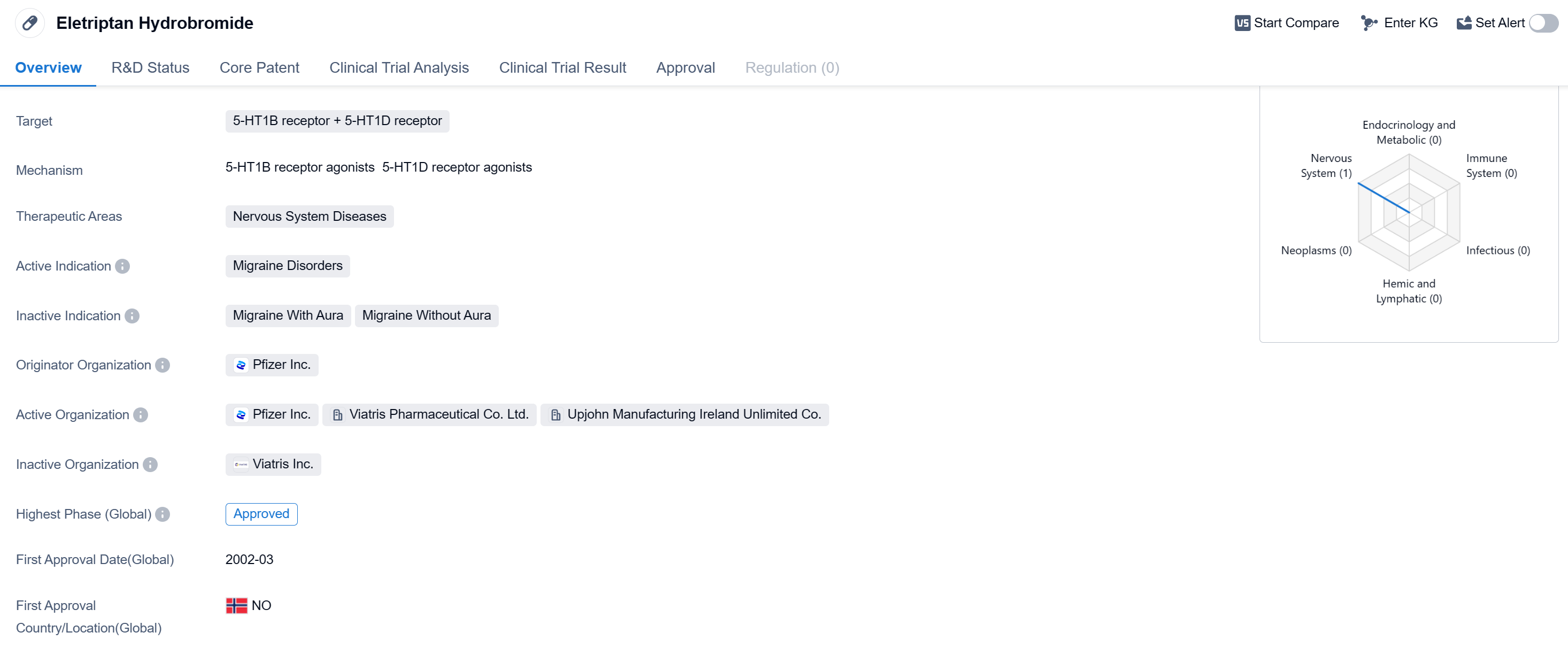
Eletriptan Hydrobromide is a small molecule drug that targets the 5-HT1B and 5-HT1D receptors. It falls under the therapeutic area of Nervous System Diseases, specifically for the treatment of Migraine Disorders. The drug was first approved globally in March 2002 and the first country to approve it was Norway.
Eletriptan Hydrobromide is developed by Pfizer Inc., a renowned pharmaceutical company. As a small molecule drug, it is designed to interact with specific receptors in the body to produce a therapeutic effect. In the case of Eletriptan Hydrobromide, it targets the 5-HT1B and 5-HT1D receptors, which are involved in the regulation of serotonin levels in the brain.
Migraine Disorders are a type of neurological condition characterized by severe headaches, often accompanied by other symptoms such as nausea, vomiting, and sensitivity to light and sound. Eletriptan Hydrobromide is indicated for the treatment of acute migraine attacks, providing relief from the symptoms and helping patients manage their condition.
The approval of Eletriptan Hydrobromide in Norway in 2002 marked an important milestone for the drug. Since then, Eletriptan Hydrobromide has been approved in other countries as well, making it available to a wider population of patients suffering from migraine disorders.
As a medication for migraine disorders, Eletriptan Hydrobromide offers an alternative treatment option for patients who experience frequent and debilitating migraine attacks. By targeting the 5-HT1B and 5-HT1D receptors, it helps to alleviate the symptoms associated with migraines and improve the quality of life for affected individuals.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Eletriptan hydrobromide: 5-HT1B receptor agonists and 5-HT1D receptor agonists
From a biomedical perspective, 5-HT1B receptor agonists and 5-HT1D receptor agonists are types of drugs that target specific receptors in the brain called 5-HT1B and 5-HT1D receptors, respectively. These receptors are part of the serotonin system, which plays a crucial role in regulating various physiological and neurological processes.
When these drugs act as agonists, they bind to and activate the 5-HT1B or 5-HT1D receptors, mimicking the effects of serotonin. By stimulating these receptors, these drugs can modulate the release and activity of neurotransmitters in the brain, particularly serotonin. This modulation can have various effects on the central nervous system, including the regulation of pain perception, mood, and vascular tone.
The activation of 5-HT1B receptors can lead to vasoconstriction (narrowing of blood vessels), which can be beneficial in treating migraines and cluster headaches. On the other hand, the activation of 5-HT1D receptors can also contribute to the alleviation of migraines and cluster headaches, as well as inhibit the release of other neurotransmitters involved in pain signaling.
Overall, 5-HT1B receptor agonists and 5-HT1D receptor agonists are pharmacological agents that selectively target specific receptors in the brain to modulate serotonin signaling. These drugs have shown efficacy in the treatment of migraines and cluster headaches, providing relief to patients experiencing these conditions.
Drug Target R&D Trends for Eletriptan hydrobromide
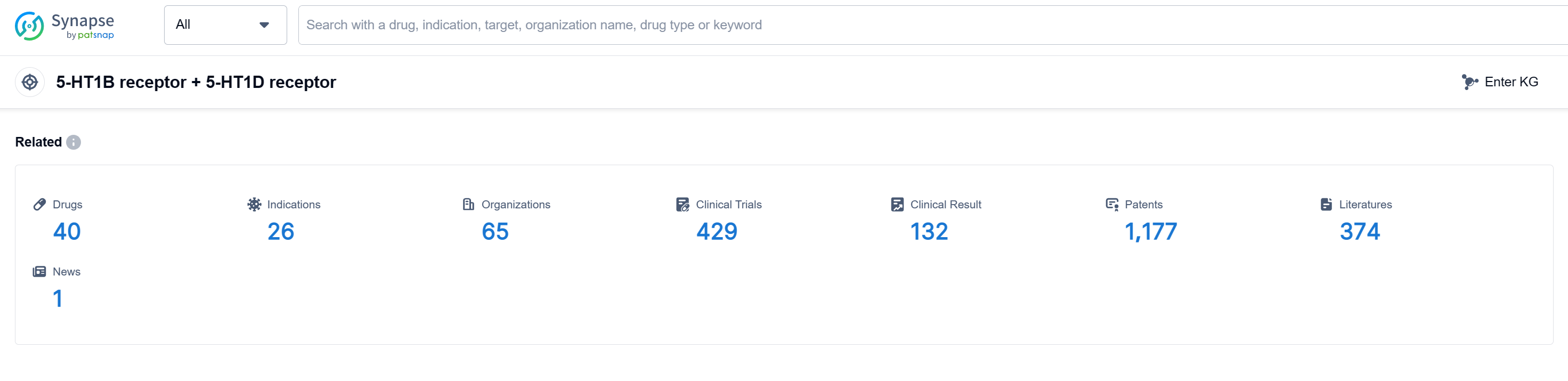
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 40 5-HT1B receptor + 5-HT1D receptor drugs worldwide, from 65 organizations, covering 26 indications, and conducting 429 clinical trials.
Based on the analysis of the provided data, the target 5-HT1B receptor + 5-HT1D receptor shows significant development and progress in the pharmaceutical industry. Companies like GSK Plc, SAWAI GROUP HOLDINGS Co., Ltd., and Eisai Co., Ltd. are leading in terms of R&D progress. The most common approved indication for drugs targeting this receptor is Migraine Disorders. Small molecule drugs dominate the drug type analysis, indicating intense competition in this area. The United States, European Union, China, and Japan are the countries/locations with the highest development progress. China has shown progress in terms of drug development for the target. Overall, the current competitive landscape for the target 5-HT1B receptor + 5-HT1D receptor is promising, and future development in this area is expected to continue.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Eletriptan Hydrobromide is a small molecule drug developed by Pfizer Inc. It targets the 5-HT1B and 5-HT1D receptors and is indicated for the treatment of acute migraine attacks. It has been available in other countries. Eletriptan Hydrobromide provides a valuable treatment option for patients suffering from migraine disorders, offering relief from symptoms and improving their overall well-being.