Gilead Issues Progress Report for EVOKE-01 Phase 3 Research Trial
Gilead Sciences, Inc. has disclosed that its EVOKE-01 Phase 3 clinical trial fell short of achieving its principal goal of extended overall survival for patients with metastatic non-small cell lung cancer who have undergone prior treatments. The EVOKE-01 research is conducting a comparative analysis of Trodelvy (sacituzumab govitecan-hziy; SG) against docetaxel in individuals with metastatic or advanced NSCLC who experienced disease progression following treatment with platinum-based chemotherapy and immunotherapy involving checkpoint inhibitors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The research revealed a statistically significant prolongation in overall survival (OS) which favored Sacituzumab Govitecan (SG), this extended to individuals with both squamous cell carcinoma and other histological types. The tolerability of Trodelvy aligned with observations from previous research. Trodelvy demonstrated acceptable levels of tolerance among patients, with no unforeseen safety concerns arising in this demographic.
A notable extension in median OS of over three months was noted in favor of SG among a certain segment of patients exhibiting resistance to their most recent anti-PD-(L)1 therapy; this subgroup constituted a majority, more than 60%, of those participating in the trial. Although this part of the analysis was pre-established in the experimental guidelines, it was conducted without strict alpha-adjustments for definitive statistical significance. This extent of difference was not mirrored in the subset of individuals who experienced a positive reaction to their last anti-PD-(L)1 treatment. Gilead is planning further investigations to elucidate how SG might benefit this patient cohort, considering there is a considerable demand for new treatment options.
Merdad Parsey, MD, PhD, Chief Medical Officer of Gilead Sciences, expressed that, "Our cumulative data continues to reinforce our optimism in Trodelvy's capacity to manage metastatic non-small cell lung cancer (NSCLC), as well as our wider efforts in lung cancer treatment trials. The hurdles in treating metastatic NSCLC that has progressed beyond platinum-based chemotherapy are noteworthy, and there remains a critical necessity for secure and potent therapeutic options. Our goal is to precisely determine which subsets of patients with metastatic NSCLC stand to gain from Trodelvy use."
Trodelvy is the pioneering Trop-2 targeting antibody-drug conjugate that has shown significant life-prolonging benefits in two distinct forms of metastatic breast cancer and has elevated treatment outcomes for certain patients with second-line (2L) metastatic urothelial cancer.
At this time, no regulatory authority has sanctioned the use of Trodelvy for metastatic NSCLC, and its safety and effectiveness for this particular use remain unverified. Trodelvy includes a Boxed Warning regarding the potential for severe or life-threatening neutropenia and severe diarrhea. For the approved U.S. Indication and more crucial safety details, please refer to the specific information provided.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
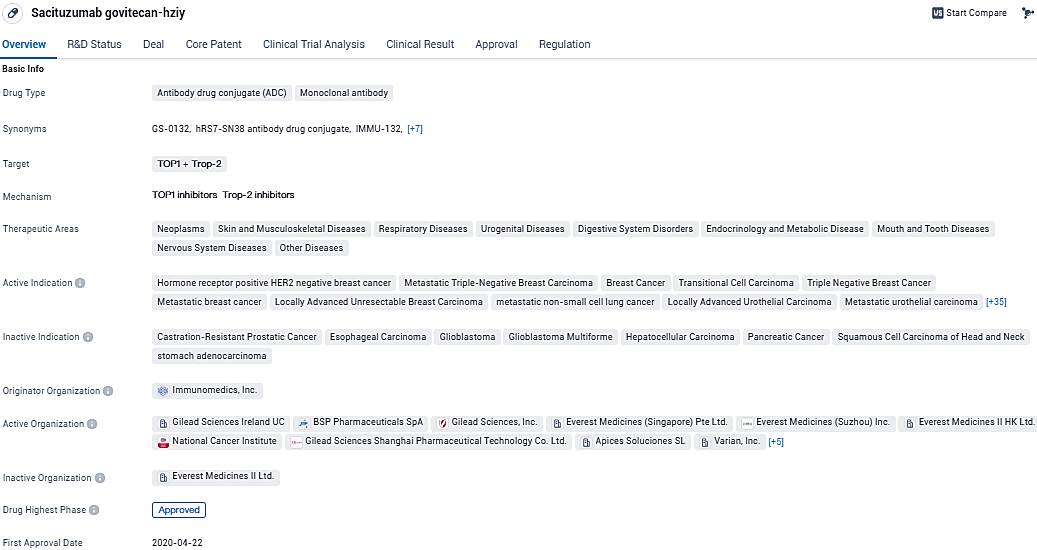
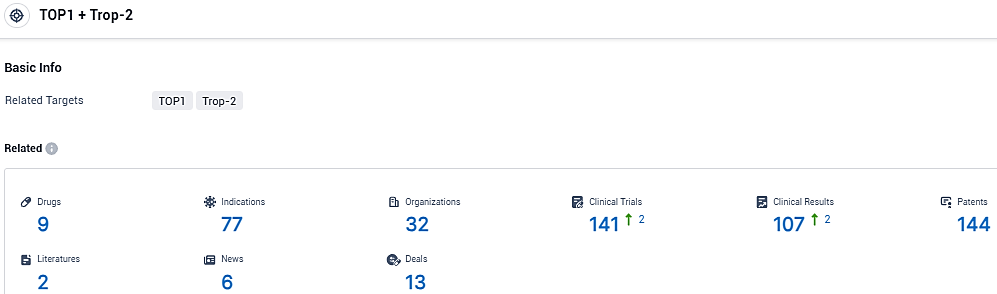
According to the data provided by the Synapse Database, As of January 26, 2024, there are 9 investigational drugs for the TOP1 and Trop-2 target, including 77 indications, 32 R&D institutions involved, with related clinical trials reaching 141, and as many as 144 patents.
Trodelvy (sacituzumab govitecan-hziy) is a first-in-class Trop-2-directed antibody-drug conjugate. Trop-2 is a cell surface antigen highly expressed in multiple tumor types, including in more than 90% of breast, bladder and lung cancers. In the U.S., Trodelvy has an accelerated approval for treatment of certain patients with second-line metastatic urothelial cancer; see below for full indication statements.