VBI Vaccines Reports New Phase 2b Results for VBI-1901 in Recurrent Glioblastoma
VBI Vaccines Inc., a biopharmaceutical firm dedicated to utilizing immunology for the prevention and treatment of diseases, has disclosed new interim results regarding tumor responses from its current randomized, controlled Phase 2b trial of VBI-1901, their experimental immunotherapeutic cancer vaccine, in patients with recurrent glioblastoma. These findings are set to be showcased during a poster session at the 2024 American Society of Clinical Oncology Annual Meeting on Saturday, June 1, 2024.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
David E. Anderson, Ph.D., Chief Scientific Officer at VBI, remarked: “The tumor responses observed so far are highly promising. Just like the results seen in the Phase 1/2a trials, I am eager to see how these findings will translate to clinical and survival outcomes by the end of this year. The current treatments available for patients with recurrent GBM have minimal to no effectiveness, reflecting data from the standard-of-care group in this study.”
Jeff Baxter, President and CEO of VBI, stated: “The data we have gathered signify substantial progress in our mission to combat GBM. We anticipate more tumor response data and initial survival metrics throughout the rest of 2024. Depending on the strength of these clinical results, we plan to start conversations with the FDA regarding an expedited development and approval pathway, leveraging our Fast Track and Orphan Drug Designations.”
Scientific literature indicates a high prevalence of CMV infection in a variety of solid tumors, including glioblastoma. GBM is one of the most prevalent and aggressive malignant primary brain tumors in humans. In the U.S. alone, there are 12,000 new cases diagnosed annually. The current treatment standard for GBM includes surgical resection followed by radiation and chemotherapy. Despite aggressive treatment, GBM progresses rapidly and has a high mortality rate.
VBI-1901 is an innovative cancer vaccine candidate developed using VBI’s enveloped virus-like particle technology to target two immunogenic cytomegalovirus antigens, gB and pp65. The FDA has granted Fast Track and Orphan Drug Designations to VBI-1901 for treating recurrent glioblastoma.
These designations offer certain benefits to drug developers, such as more frequent FDA meetings, Accelerated Approval, and Priority Review, assuming relevant criteria are fulfilled.
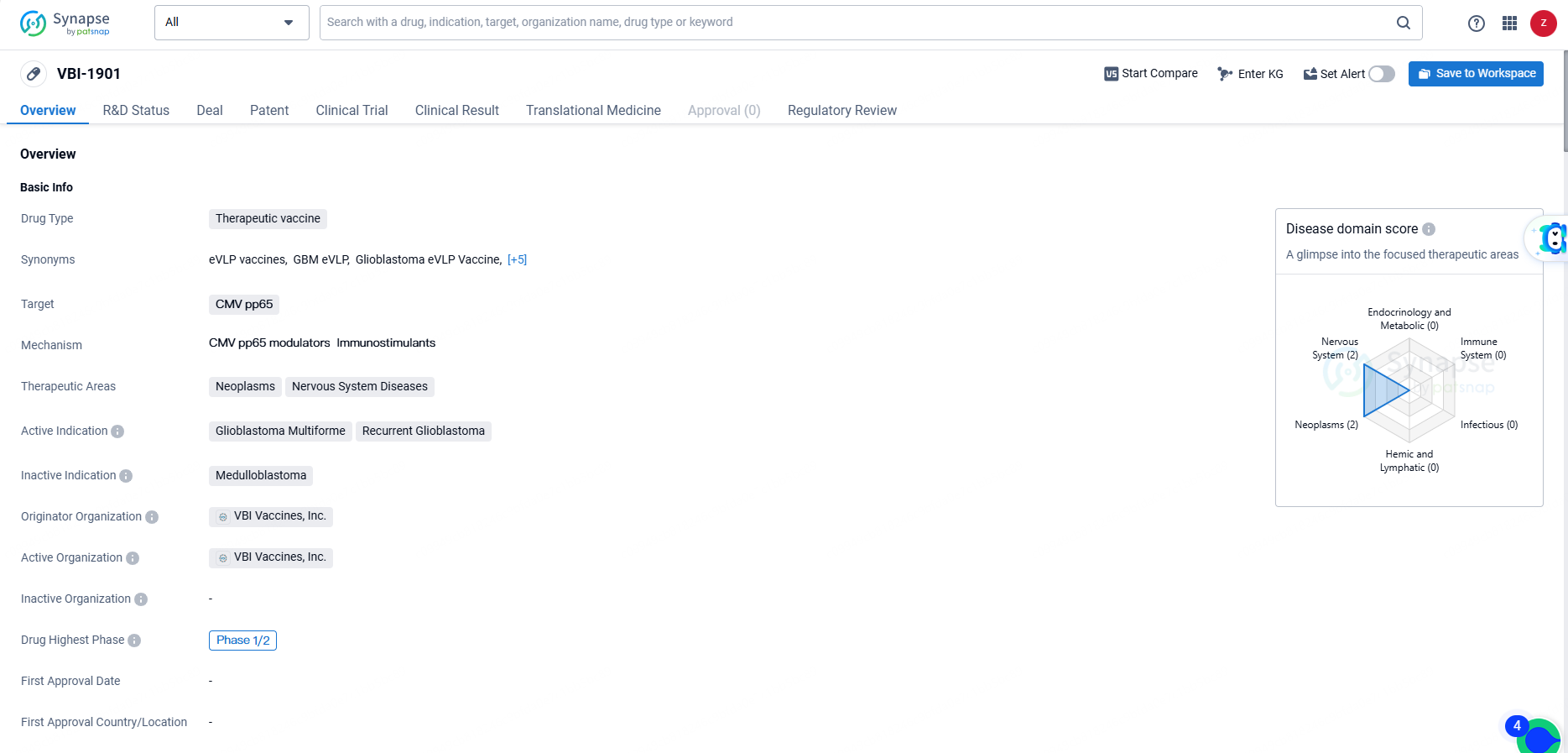
VBI-1901 is a therapeutic vaccine created by VBI Vaccines, Inc. The drug targets CMV pp65, aiming to treat neoplasms and nervous system diseases, particularly focusing on Glioblastoma Multiforme and Recurrent Glioblastoma. It is currently in Phase 1/2 of development, indicating its potential in early clinical trials.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
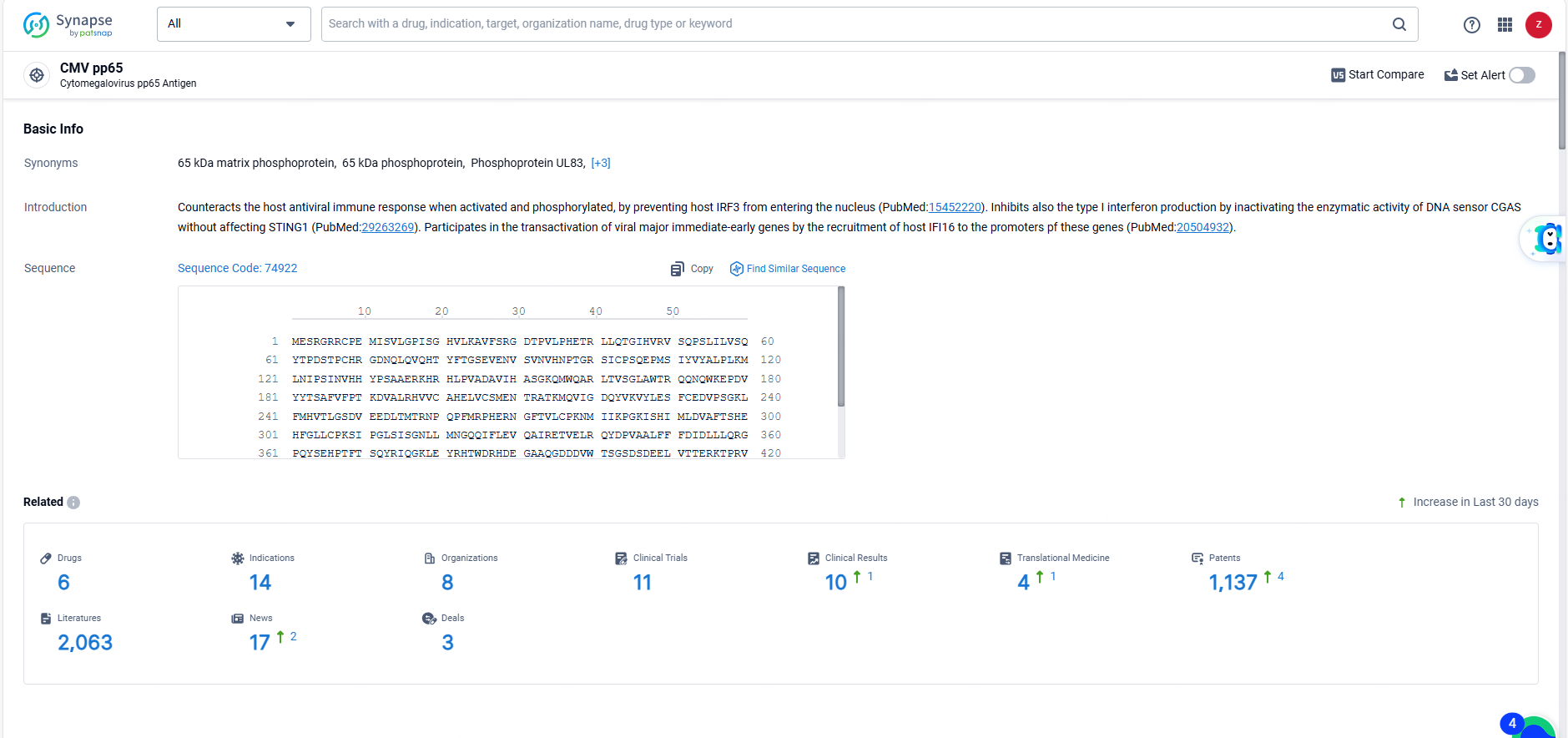
According to the data provided by the Synapse Database, As of June 3, 2024, there are 6 investigational drugs for the CMV pp65 target, including 14 indications, 8 R&D institutions involved, with related clinical trials reaching 11, and as many as 1137 patents.
VBI-1901 specific targeting of CMV pp65 and its focus on neoplasms and nervous system diseases indicate a targeted approach to addressing these conditions. The Fast Track and Orphan Drug designations further highlight the potential significance of VBI-1901 in addressing unmet medical needs.