Verismo Therapeutics gets FDA's IND approval for SynKIR™-310 in treating relapsed/refractory B-cell NHL
Verismo Therapeutics, a biopharmaceutical company in the clinical stage focusing on CAR T innovations, has revealed that the US Food and Drug Administration has given the green light for the Phase 1 clinical trial of SynKIR™-310 aimed at evaluating its effectiveness for NHL.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The CELESTIAL-301 Phase 1 trial will evaluate the safety, tolerability, and preliminary effectiveness of SynKIR™-310 in patients with relapsed or refractory B-cell Non-Hodgkin Lymphomas, specifically Diffuse Large B Cell Lymphoma, Follicular Lymphoma, Mantle Cell Lymphoma, and Marginal Zone Lymphoma. This trial will include patients who have relapsed or become refractory after CAR T cell therapy as well as those who have not previously undergone CAR T cell treatment.
The CELESTIAL-301 study targets several critical areas of unmet medical need. Although commercial CAR T cell therapies initially demonstrate high response rates in hematologic malignancies, relapse occurs in about 40-50% of cases over time. These relapses are partly due to the lack of sustained T cell effector function and persistence.
For patients with relapsed/refractory DLBCL who relapse post-commercial CAR T cell treatment, current treatment options are highly limited. Ongoing clinical studies have not yet fully addressed the unmet needs of these patients.
SynKIR™-310, developed using Verismo’s KIR-CAR platform and proprietary CD19 binder, addresses B-cell associated conditions and malignancies. The binder, specifically designed for the KIR-CAR platform, aims to treat B-cell disorders more effectively. SynKIR™-310, guided by DS191, targets a similar CD19 epitope as existing CAR T therapies but aims to enhance the longevity and function of the anti-tumor T cells. Dr. Donald Siegel and Dr. Michael Milone, co-founders of Verismo, are the inventors of this binder.
Verismo intends to commence the CELESTIAL-301 clinical trial in the latter half of 2024. This trial will be the company’s second clinical study focusing on the KIR-CAR platform technology, which leverages natural NK cell receptors to potentially revolutionize T cell therapies.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
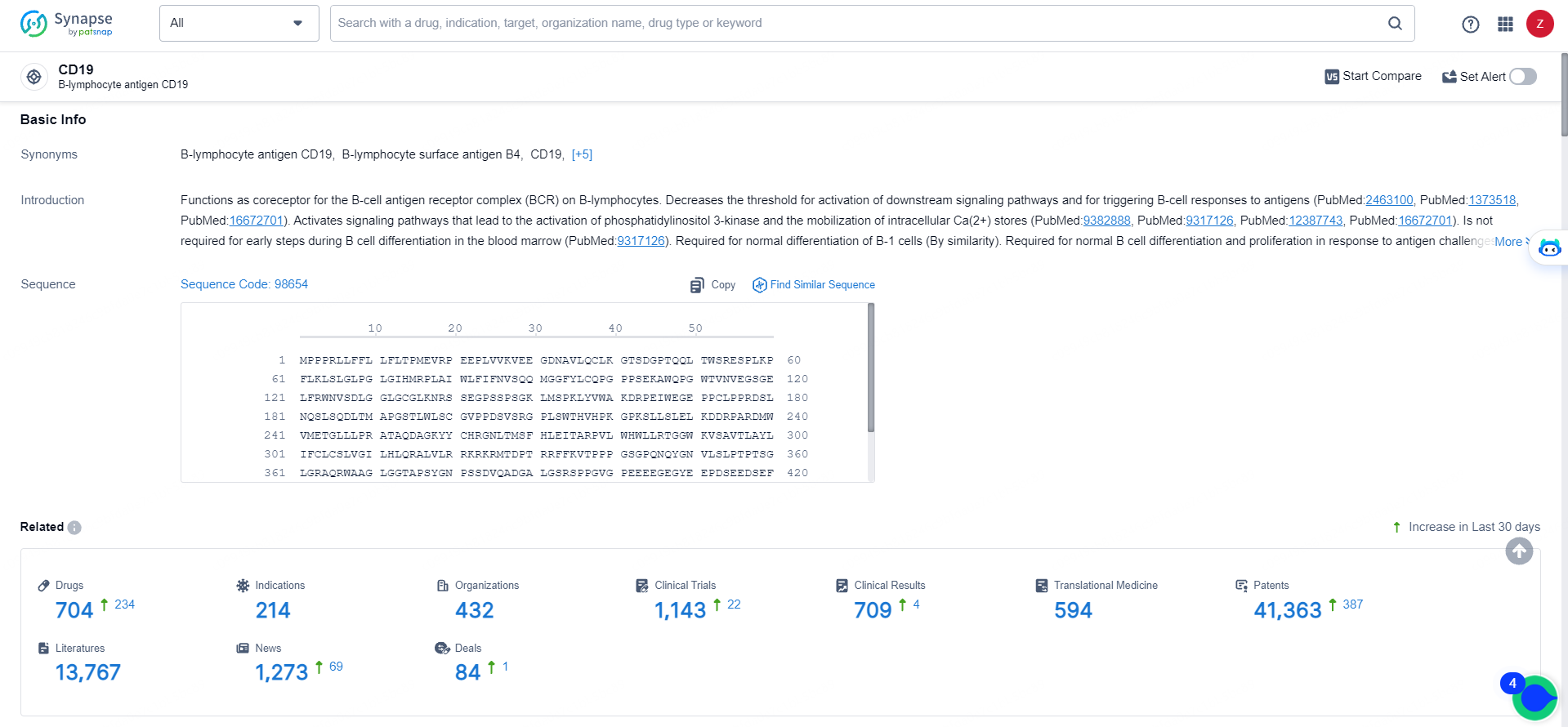
According to the data provided by the Synapse Database, As of May 16, 2024, there are 704 investigational drugs for the CD19 targets, including 214 indications, 432 R&D institutions involved, with related clinical trials reaching 1143, and as many as 41363 patents.
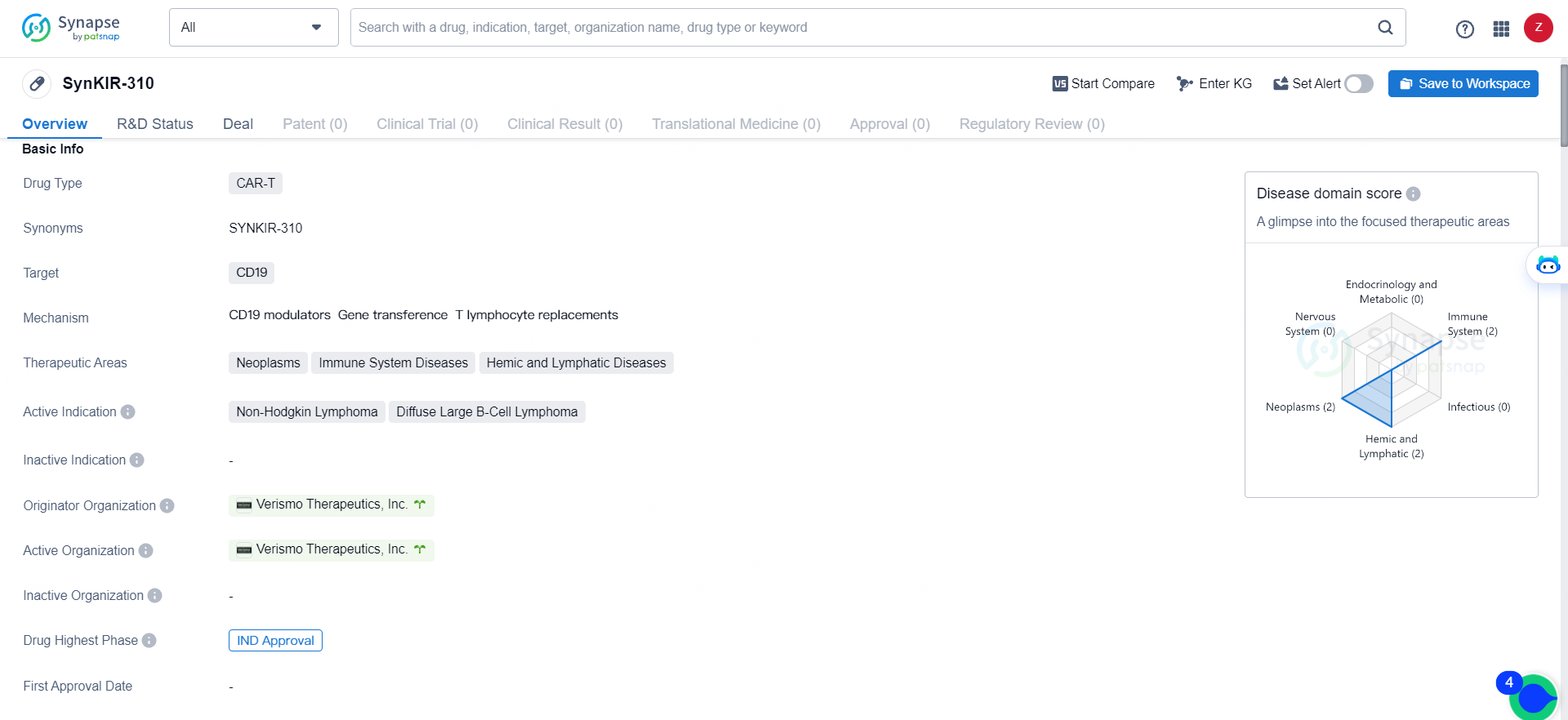
SynKIR-310 targets CD19 and is indicated for the treatment of Non-Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma. It falls under the therapeutic areas of Neoplasms, Immune System Diseases, and Hemic and Lymphatic Diseases. SynKIR-310 represents an innovative approach to cancer treatment, leveraging the body's own immune system to combat the disease. As it progresses through clinical development, it has the potential to offer new hope to patients with Non-Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma.