Aviceda Therapeutics Reports Promising Early Data from AVD-104 Study in Geographic Atrophy
Aviceda Therapeutics has released encouraging initial results from the first segment of its Phase 2/3 SIGLEC study focused on AVD-104 for the treatment of patients suffering from geographic atrophy, which is a consequence of age-related macular degeneration. The findings indicate that after a single administration of AVD-104, favorable results concerning the agent's safety and effectiveness were evident within a three-month timeframe.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"We are thrilled by the indications of substantial visual and functional enhancements along with a swift decline in the progression rate of GA lesions after administering a single dose of AVD-104," stated David Callanan, M.D., the Chief Medical Officer and Senior Vice President of Aviceda. "Our anticipation is high for the commencement of SIGLEC's Part 2, where we aim to validate AVD-104's potential to offer a significant advancement compared to treatments solely relying on complement inhibition."
"Observing the progression of a unique therapeutical strategy for GA, our optimism is bolstered by the positive outcomes witnessed in SIGLEC's initial segment. We are of the belief that AVD-104's synergistic mechanism of action, addressing both macrophage/microglial- driven and complement-induced inflammation, might redefine GA treatment standards," expressed Mohamed Genead, M.D., who is a Co-founder and the CEO.
Mohamed Genead went on to specify, "We deem it vital to efficiently target both cellular and humoral elements that propel disease advancement specifically in the junctional zone, the frontline of GA attack. The insights from Part 1 of SIGLEC substantiate AVD-104's potential in this important aspect."
Participants in the first phase of the study successfully received a one-time administration of AVD-104 with a follow-up at month 3. There were no observations of serious adverse effects associated with the drug, either systemically or related to ocular health. A notable deceleration in the progression of GA lesions at the 3-month mark was noted in comparison to past outcomes with standard care. There was a notable improvement in vision, gauged by the best corrected visual acuity, subsequent to the initial injection of AVD-104, and this improvement persisted through month 3.
SIGLEC's Part 2 will be a pivotal study, involving multiple centers, conducted under double-masked conditions, and will be a randomized and controlled examination to discern the efficacy and safety of AVD-104 against an active therapy for GA attributable to AMD. The trial is set to include roughly 300 subjects and will offer a continuation within the study for an added year. The primary goal will be to ascertain the variance in GA lesion expansion rate between the treated individuals and those receiving the active comparison over a span of 12 months, with fundus autofluorescence serving as the measuring tool.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
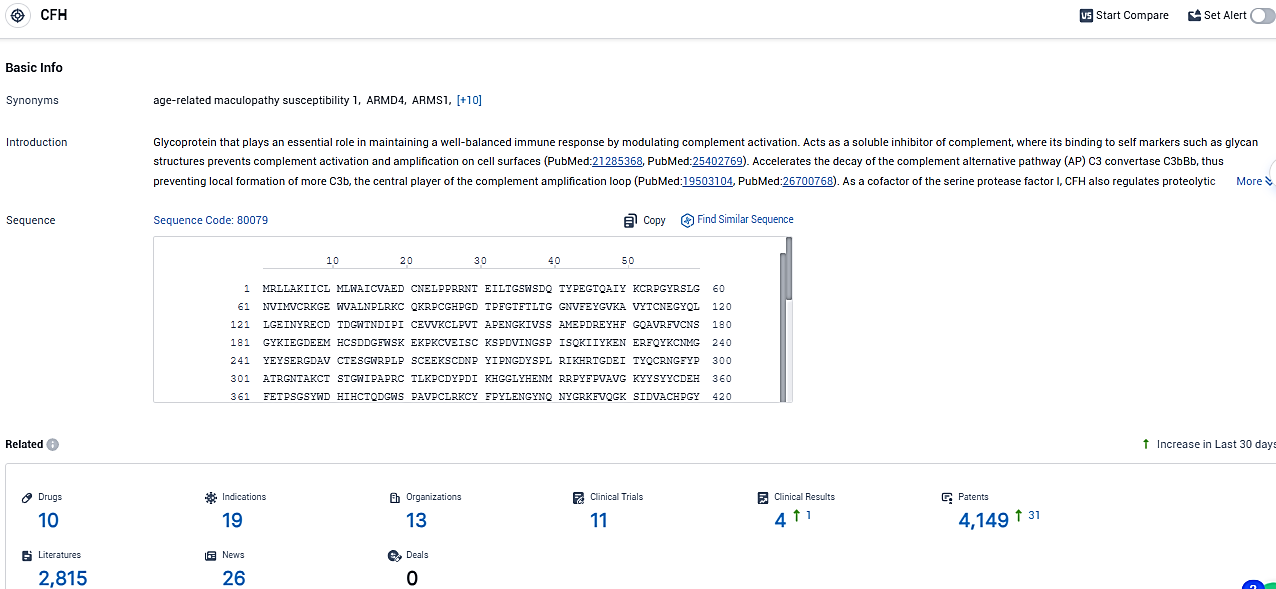
According to the data provided by the Synapse Database, As of January 23, 2024, there are 10 investigational drugs for the CFH target, including 19 indications, 13 R&D institutions involved, with related clinical trials reaching 11, and as many as 4149 patents.
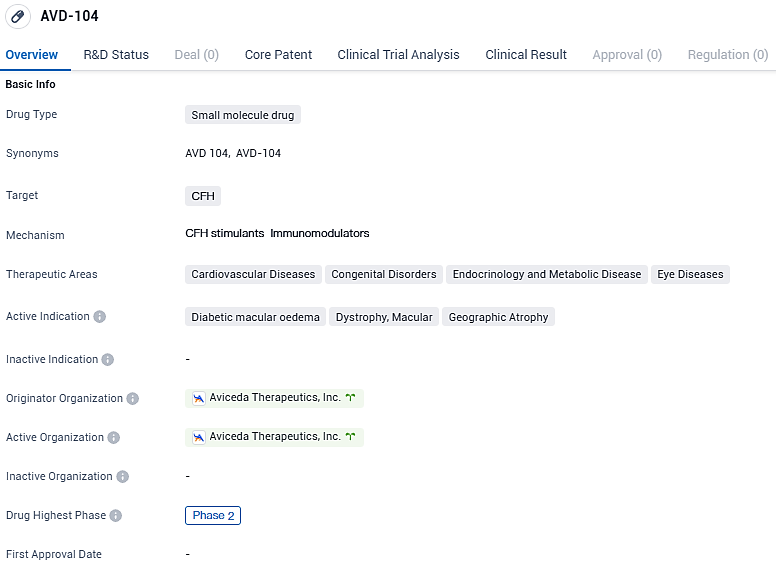
AVD-104 targets the CFH protein and shows promise in treating various therapeutic areas, including cardiovascular diseases, congenital disorders, endocrinology and metabolic diseases, as well as eye diseases. The drug's active indications include diabetic macular oedema, dystrophy macular, and geographic atrophy. Currently in Phase 2 of clinical development, AVD-104 is undergoing further evaluation to determine its efficacy and safety profile.