Viking Therapeutics Announces Results from First Human Trial of Oral GLP-1/GIP Dual Agonist VK2735
Viking Therapeutics, Inc., has released encouraging outcomes from its Phase 1 clinical study, which tested multiple increasing doses of an orally administered version of VK2735. This investigational drug is designed to target and activate both the glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors. It's primarily aimed at tackling metabolic conditions, including obesity. With the successful data obtained from the initial phase, Viking Therapeutics is poised to move forward with a Phase 2 study of the oral VK2735 formulation in patients with obesity in the latter part of this calendar year.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The findings from the 28-day Multiple Ascending Dose (MAD) investigation indicate encouraging indicators of clinical efficacy with VK2735 administered orally. Groups treated with VK2735 showed a decrease in average body weight starting from the initial measurements, with the maximum drop recorded at 5.3%. Additionally, those treated with VK2735 exhibited weight loss compared to the placebo-controlled group, with figures reaching 3.3%. For dosages of VK2735 equal to or greater than 10 mg, the average weight decrease as compared to placebo remained consistent or even showed improvement on Day 34, which is six days subsequent to the final VK2735 dose, with a maximum of 3.6% difference versus placebo.
In a preliminary exploration concerning the percentage of participants who experienced a minimum of 5% decrease in weight after the 28-day period, it was revealed that a high of 57% of individuals receiving VK2735 attained this degree of weight loss, in contrast with none in the placebo group. Analyses of the patterns in weight loss prompt the company to infer that extending the treatment period past 28 days could lead to more pronounced weight reduction.
Brian Lian, Ph.D., and CEO of Viking Therapeutics, emphasized, "The early outcomes from the Phase 1 study underscore the encouraging potential of VK2735 regarding initial weight loss and its capability to be well-tolerated as an orally ingested tablet. Our interpretation of the findings suggests that an extended treatment timeline with potentially increased dosage could enhance weight loss." He further commented, "The preliminary safety and tolerability profiles are proving to be noteworthy, especially pertaining to the minimal gastrointestinal side effects observed. An orally administered medication that is well-received by the body could emerge as a compelling option for treating patients with obesity. We are eager to proceed with longer treatment periods and possibly higher dosages in a forthcoming Phase 2 study."
In light of these initial optimistic outcomes relating to weight reduction, as well as safety and digestibility, the company has decided to proceed with additional dose intensification in this ongoing research. Additionally, Viking is outlining plans for a commencement of a Phase 2 clinical trial focusing on VK2735 for obese patients, which is anticipated to start in the latter half of 2024.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
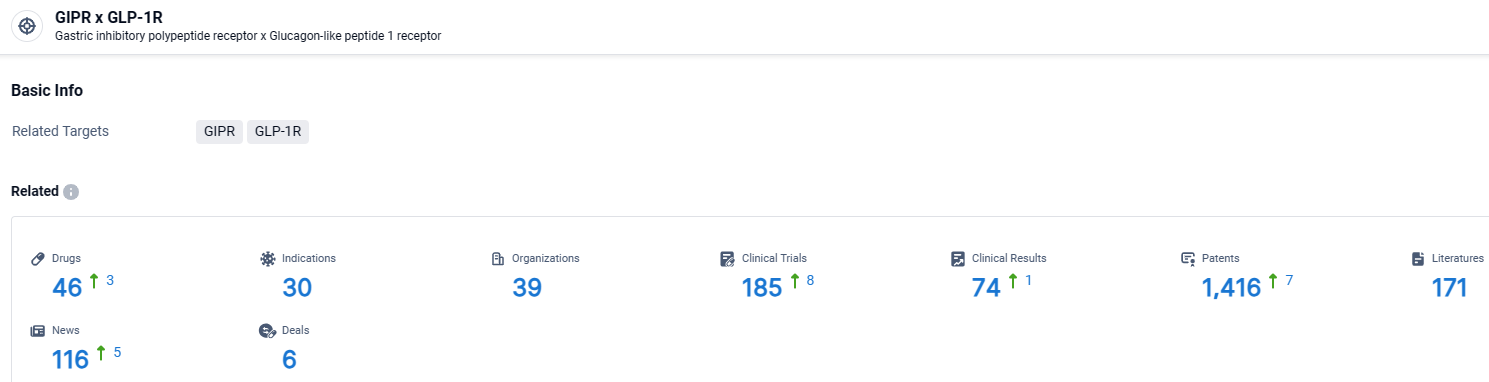
According to the data provided by the Synapse Database, As of March 29 2024, there are 46 investigational drugs for the GIPR and GLP-1R target, including 30 indications, 39 R&D institutions involved, with related clinical trials reaching 185, and as many as 1416 patents.
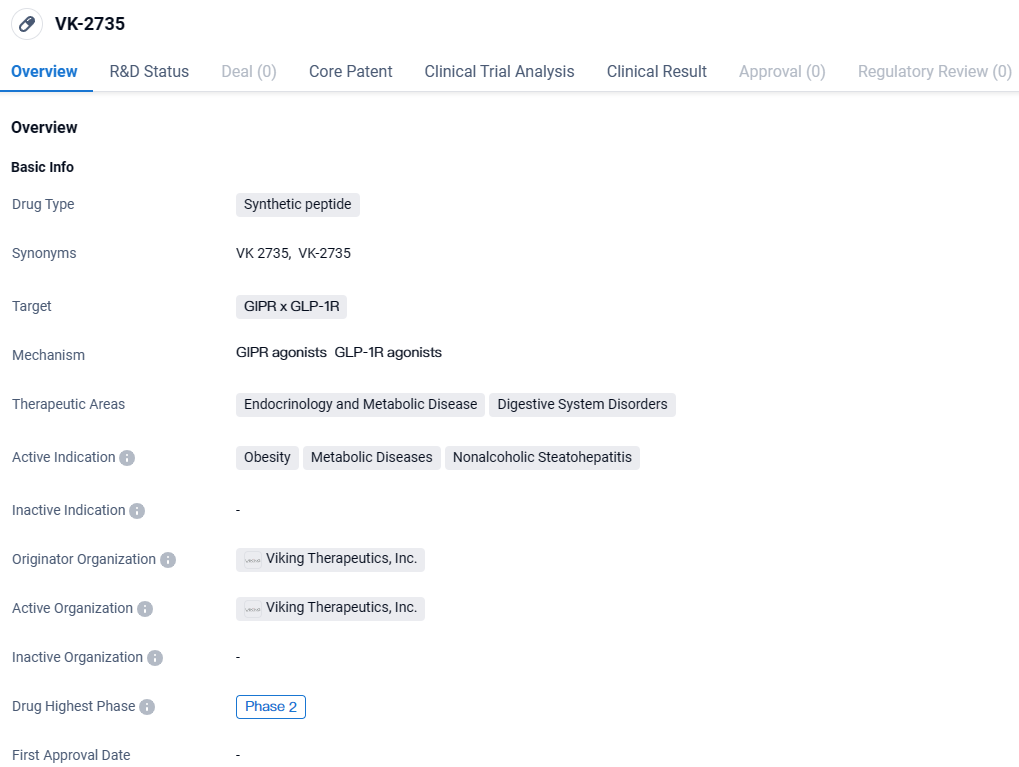
VK-2735 targets GIPR and GLP-1R. It is being developed for the treatment of obesity, metabolic diseases, and nonalcoholic steatohepatitis. With its current highest phase being Phase 2, VK-2735 shows promise in addressing significant health challenges in the field of endocrinology and metabolic diseases.