Viking Therapeutics: Focused on Metabolic and Endocrine Diseases
On June 24, the U.S. biotechnology company Viking Therapeutics showcased a series of internally developed Dual Agonists of Amylin and Calcitonin Receptors (DACARs) preclinical data at this year's Annual Scientific Meeting of the American Diabetes Association. The research results indicate that Viking's series of dual agonists have reduced food intake in lean rats within 0-72 hours after a single subcutaneous administration. After 72 hours of a single subcutaneous dose, Viking's novel compounds achieved a weight loss of up to 8% compared to the animals treated with carriers. Other highlights include:
1.Viking's DACRAs displayed EC50 values ranging from low nanomolar to micromolar on human Amylin 3 receptor, and a similar potency range on the human Calcitonin receptor.
2.Up to 8% of average weight loss was achieved in lean rats 72 hours after single-dose treatment with Viking's DACRAs.
3.After 24 days of treatment with Viking's DACRA compounds in DIO mice, a weight loss of up to 10% was shown compared to baseline (p<0.05).
4.Viking's DACRA compounds reduced blood sugar by up to 24% in DIO mice after 24 days (p<0.05, compared with baseline and cagrilintide control group).
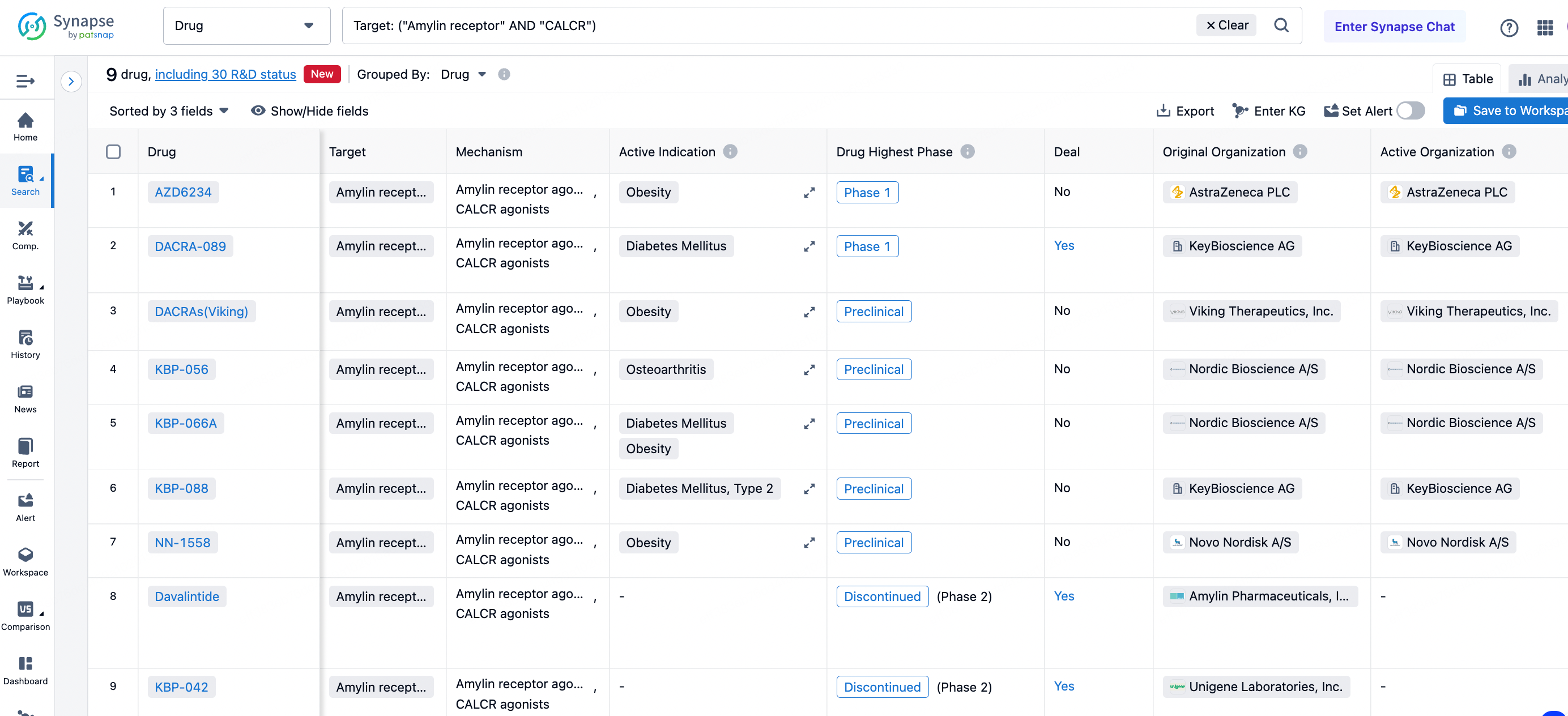
According to statistics from the Synapse database, the leading research institutions globally for Dual Agonists of Amylin and Calcitonin Receptors are AZ, with related small molecule drugs being in phase one clinical trials for obesity indications. Furthermore, the DACRA-089 from Swiss biotech firm KeyBioscience is also in phase one clinical research interpretation.
Viking Therapeutics is a clinical-stage biopharmaceutical company focused on developing novel, first-in-class or best-in-class therapies for metabolic and endocrine disorders. Currently, three compounds are undergoing clinical trials.
Viking is also developing VK2735, a novel dual agonist for the Glucagon-Like Peptide-1 (GLP-1) and Glucose-dependent Insulinotropic Polypeptide (GIP) receptors, for the potential treatment of various metabolic disorders. Data from Phase 1 and Phase 2 trials evaluating VK2735 (subcutaneously administered) in the treatment of metabolic disorders has shown promising safety and tolerability, and positive signs of clinical benefit. The company is also evaluating an oral form of VK2735 in Phase 1 trials.
Another clinical program of Viking includes VK2809, a novel, orally administered, selective thyroid hormone receptor beta agonist, for the treatment of hyperlipidemia and metabolic disorders. The compound successfully met its primary and secondary endpoints in a recently completed Phase 2b study in biopsy-confirmed non-alcoholic steatohepatitis (NASH) and fibrosis. In a Phase 2a trial targeting non-alcoholic fatty liver disease (NAFLD) and elevated LDL-C, patients treated with VK2809 showed statistically significant reductions in LDL-C and liver fat content compared to those treated with placebo.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!