US Phase 2 Trial of Akeso’s Ligufalimab-Azacitidine Combo for Myelodysplastic Syndrome Begins
Akeso has reported the successful first patient enrollment in the United States for its phase II clinical trial, featuring their novel CD47 monoclonal antibody, ligufalimab (AK117), used alongside azacitidine for patients who are newly diagnosed with higher-risk myelodysplastic syndrome.
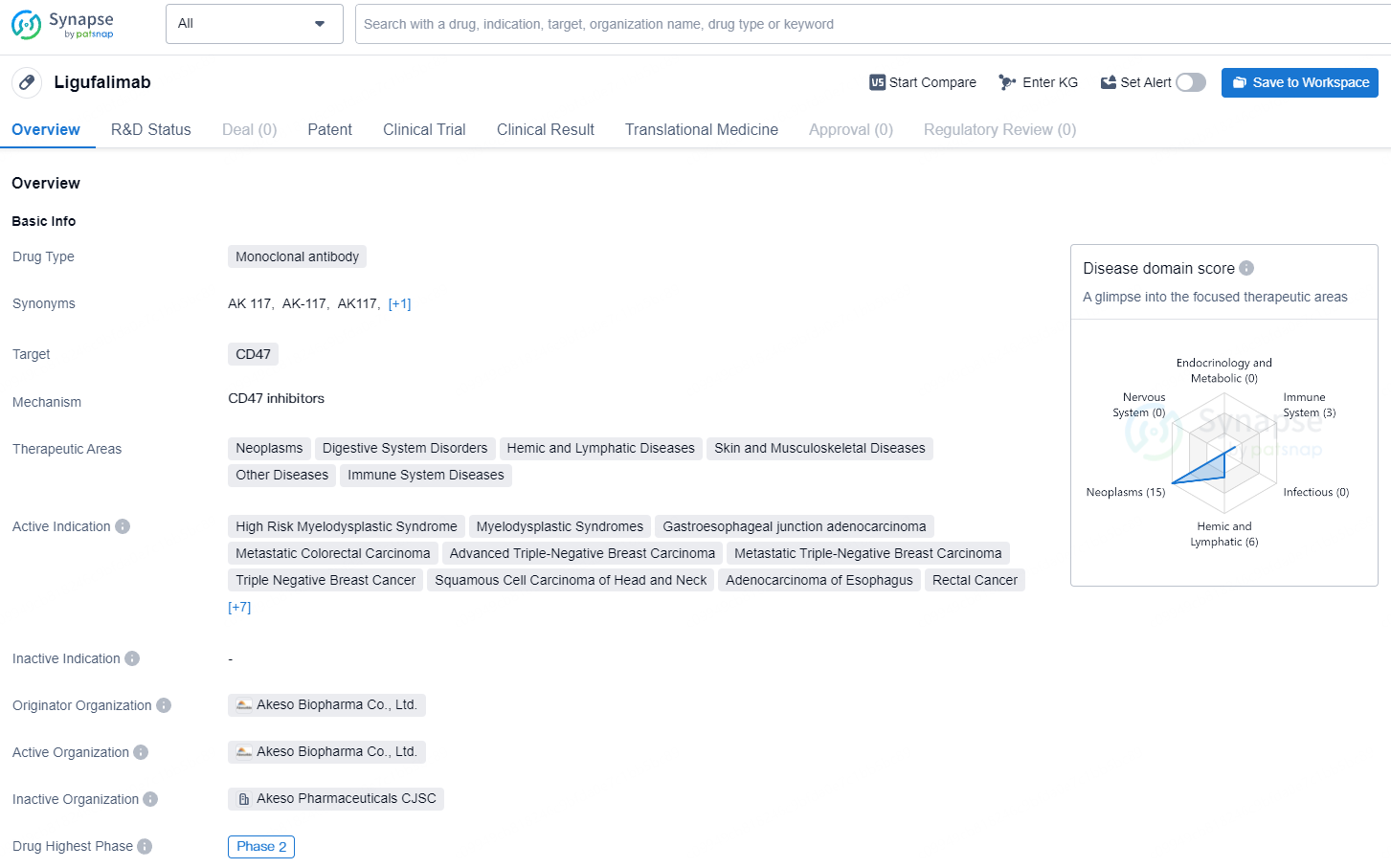
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Early investigations indicate that combining AK117 with azacitidine for the treatment of MDS demonstrates both safety and significant efficacy. Addressing the urgent demand for new therapies for MDS patients globally and the shifting market environment, Akeso has initiated a global Phase II multicenter clinical trial. This effort intends to accelerate the global approval and commercialization of AK117.
The development of CD47-targeted drugs for MDS treatment shows substantial promise. AK117, an advanced humanized IgG4 anti-CD47 antibody, effectively inhibits the CD47-SIRPα interaction, boosting phagocytes' ability to engulf tumor cells.
Recently, data presented at the 65th Annual Meeting of the American Society of Hematology highlighted that AK117, when used with azacitidine, significantly reduces anemia and lowers transfusion needs in MDS patients, demonstrating good safety and notable effectiveness. This makes AK117 a potential new treatment option for MDS patients worldwide.
In the United States alone, approximately 40,000 new MDS cases are diagnosed annually. High-risk MDS patients generally start with azacitidine as the standard treatment, but only 20% to 30% achieve complete remission, indicating a significant unmet need in MDS treatments globally.
In addition to the ongoing global clinical trials for MDS, a Phase II study is evaluating AK117 combined with venetoclax and AZA as a frontline treatment for AML patients who are ineligible for intensive chemotherapy.
Moreover, Akeso is vigorously advancing the global market development of AK117 for solid tumors. Multiple clinical trials are actively enrolling to assess the efficacy of AK117 in combination with other agents, such as PD-1/VEGF bispecific antibodies and PD-1/CTLA-4 bispecific antibodies.
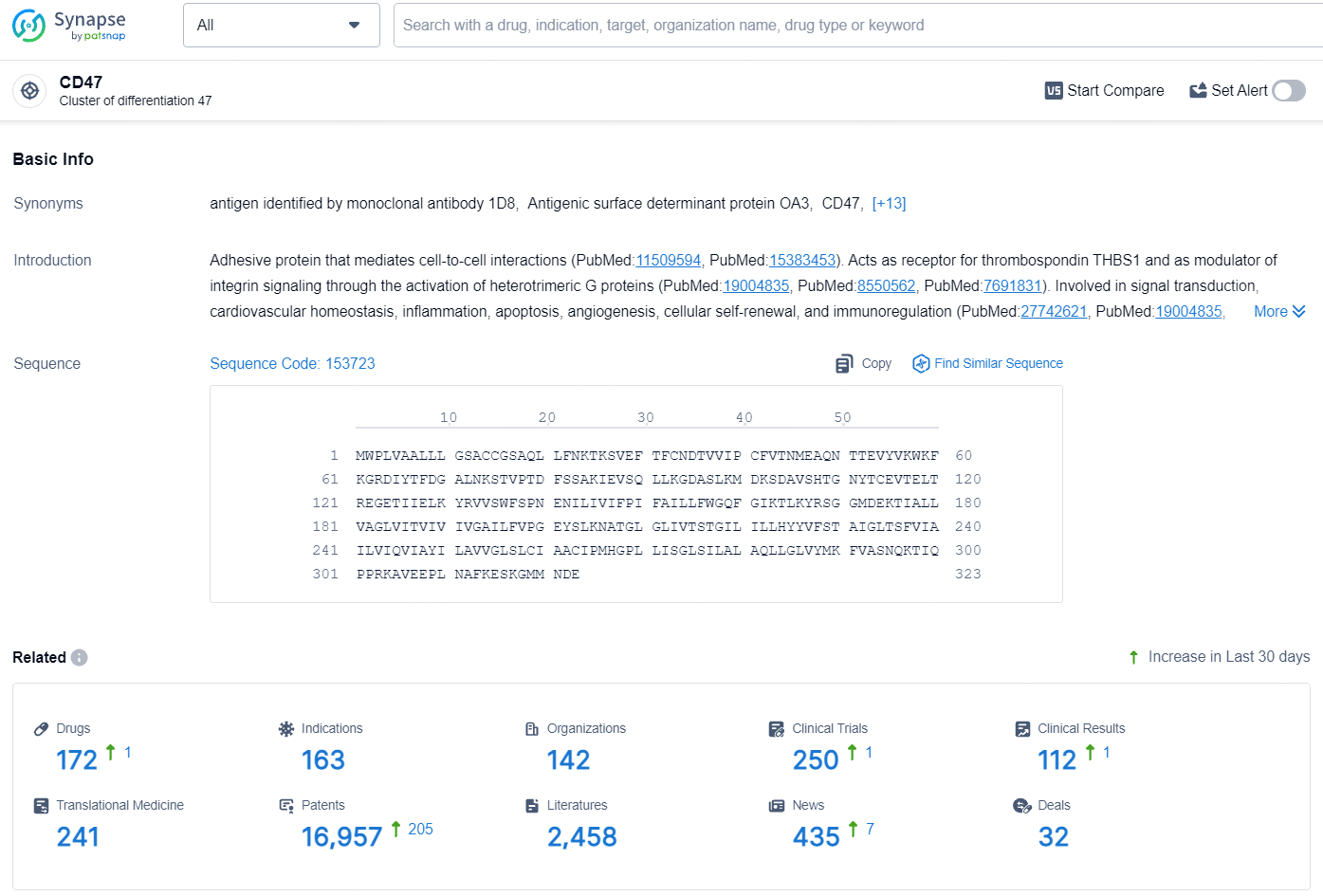
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 8, 2024, there are 172 investigational drugs for the CD47 target, including 163 indications, 142 R&D institutions involved, with related clinical trials reaching 250, and as many as 16957 patents.
Several phase II clinical trials are underway to investigate the potential of AK117 in combination with azacitidine for hematological tumors, as well as AK117 alone or in combination with ivonescimab and cadonilimab for various solid tumors. Preliminary studies have shown promising efficacy and safety profiles, with no observed dose-limiting toxicity events. Additionally, international multicenter clinical studies evaluating AK117 for treating MDS and AML are enrolling patients.