Wave Life Sciences Achieves First Successful Human RNA Editing in RestorAATion-2 Trial of WVE-006 for Alpha-1 Antitrypsin Deficiency
Wave Life Sciences Ltd. (Nasdaq: WVE), a biotechnology firm in the clinical stage devoted to exploring the extensive possibilities of RNA therapeutics for improving health outcomes, has reported favorable proof-of-mechanism results from the ongoing Phase 1b/2a RestorAATion-2 trial evaluating WVE-006 in patients with alpha-1 antitrypsin deficiency (AATD). WVE-006 is an A-to-I RNA editing oligonucleotide (AIMer) that is delivered subcutaneously and conjugated with GalNAc, developed using Wave’s advanced oligonucleotide chemistry platform. This treatment is specifically designed to target lung disease, liver disease, or both conditions associated with AATD.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The proof-of-mechanism findings released today represent the inaugural clinical evidence of RNA editing in humans. This information derives from the initial single-dose cohort (200 mg) in the RestorAATion-2 trial, which includes the first two patients diagnosed with “ZZ” AATD (Pi*ZZ AATD) to reach day 57. Patients with Pi*ZZ AATD are unable to produce wild-type alpha-1 antitrypsin (M-AAT) protein; hence, the detection of M-AAT protein indicates successful editing of mutant Z-AAT mRNA. Moreover, achieving 50% M-AAT is aligned with the heterozygous “MZ” genotype, which poses a lower risk for lung and liver diseases associated with AATD.
At day 15, the concentration of circulating wild-type M-AAT protein in plasma averaged 6.9 micromolar, accounting for more than 60% of the total AAT present. The observed rise in neutrophil elastase inhibition from baseline supports the production of active M-AAT. The average total AAT protein increased from below the quantification threshold at baseline to 10.8 micromolar by day 15, which meets the regulatory approval threshold for AAT augmentation therapies. Increases in both total AAT and M-AAT protein were recorded early, as soon as day 3, and continued through day 57.
WVE-006 has demonstrated good tolerability and a strong safety profile so far. All adverse reactions reported in the RestorAATion-2 trial, as well as in the ongoing RestorAATion-1 trial with healthy participants, were classified as mild to moderate, with no serious adverse events documented. The RestorAATion-2 study is still in progress, and Wave anticipates presenting multidose data in 2025.
“Achieving the first therapeutic RNA editing in humans marks a major achievement for our organization, our collaboration with GSK, and the entire field of oligonucleotides. It also validates and enhances the risk profile of Wave’s RNA editing technology, considering the strong clinical translation of our proprietary top-tier chemistry, including PN, stereochemistry, and our N3U AIMer modification,” stated Paul Bolno, MD, MBA, President and CEO of Wave Life Sciences. “The extent of mRNA editing we are witnessing following a single dose has surpassed our expectations, and we predict M-AAT levels will keep rising with additional doses, based on our preclinical insights. These preliminary results, in conjunction with the durability of WVE-006 and its ease of subcutaneous administration, support its classification as a best-in-class candidate compared to alternative editors and within the broader AATD landscape. This data enhances our confidence in our fully owned pipeline, including initiatives for HD, DMD, and obesity, alongside upcoming RNA editing targets. We are eager to unveil our next RNA editing initiatives, as well as updates on our INHBE GalNAc-siRNA program targeting obesity, at our Research Day scheduled for October 30.”
In the US and Europe, an estimated 200,000 individuals carry the homozygous SERPINA1 Z mutation resulting in AATD. Current treatment options are restricted to weekly intravenous augmentation therapy aimed at lung disease, generating over $1.4 billion in global sales in 2023. There are no approved treatments for AATD-related liver disease, a condition that often necessitates liver transplants for many affected individuals.
GSK holds the exclusive global rights to WVE-006. After Wave completes the RestorAATion-2 trial, the responsibilities for its development and commercialization will transition to GSK. Wave stands to gain up to $525 million in milestone payments, in addition to tiered royalties based on net sales of WVE-006.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
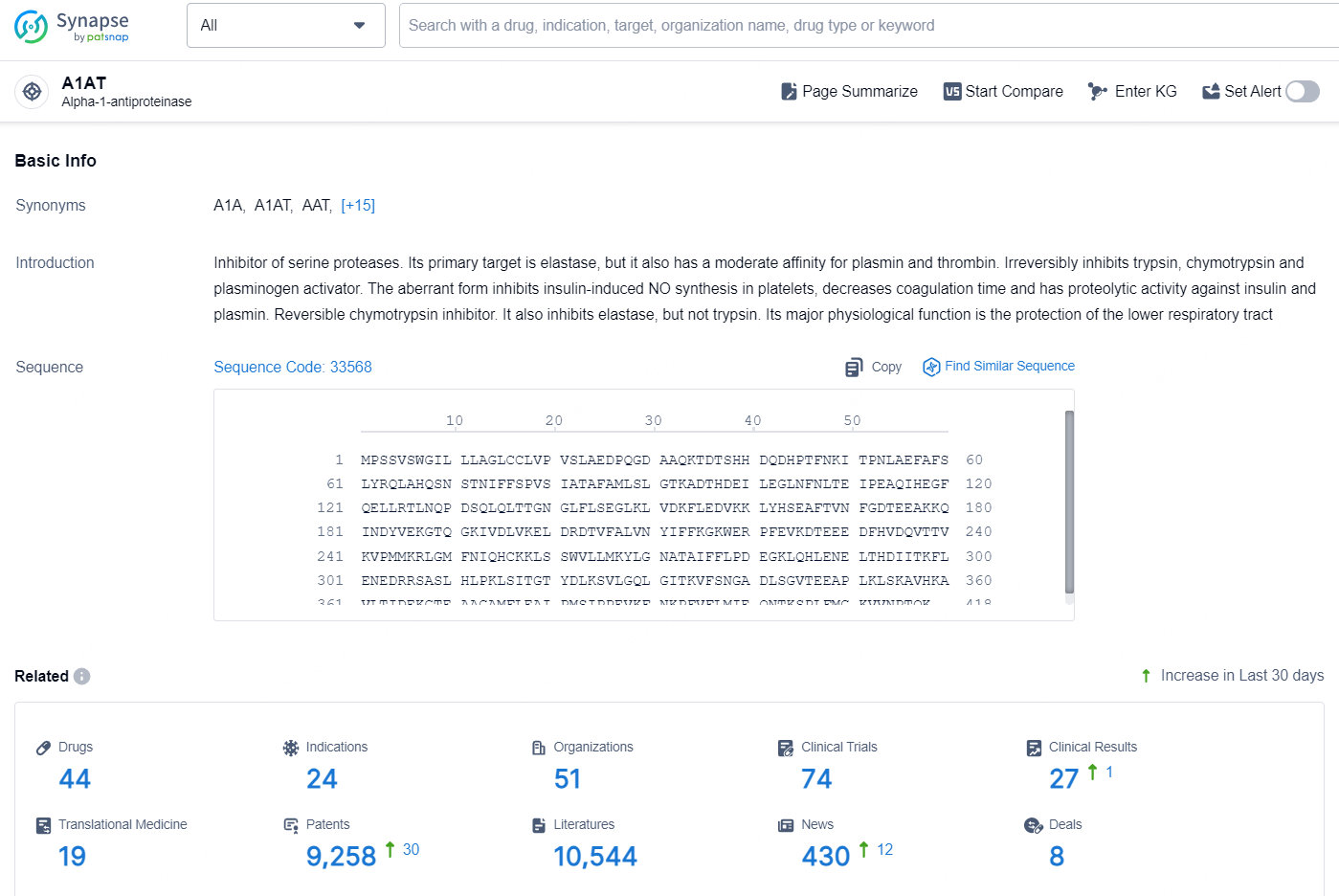
According to the data provided by the Synapse Database, As of October 21, 2024, there are 44 investigational drugs for the A1AT target, including 24 indications, 51 R&D institution involved, with related clinical trial reaching 74, and as many as 9258 patents.
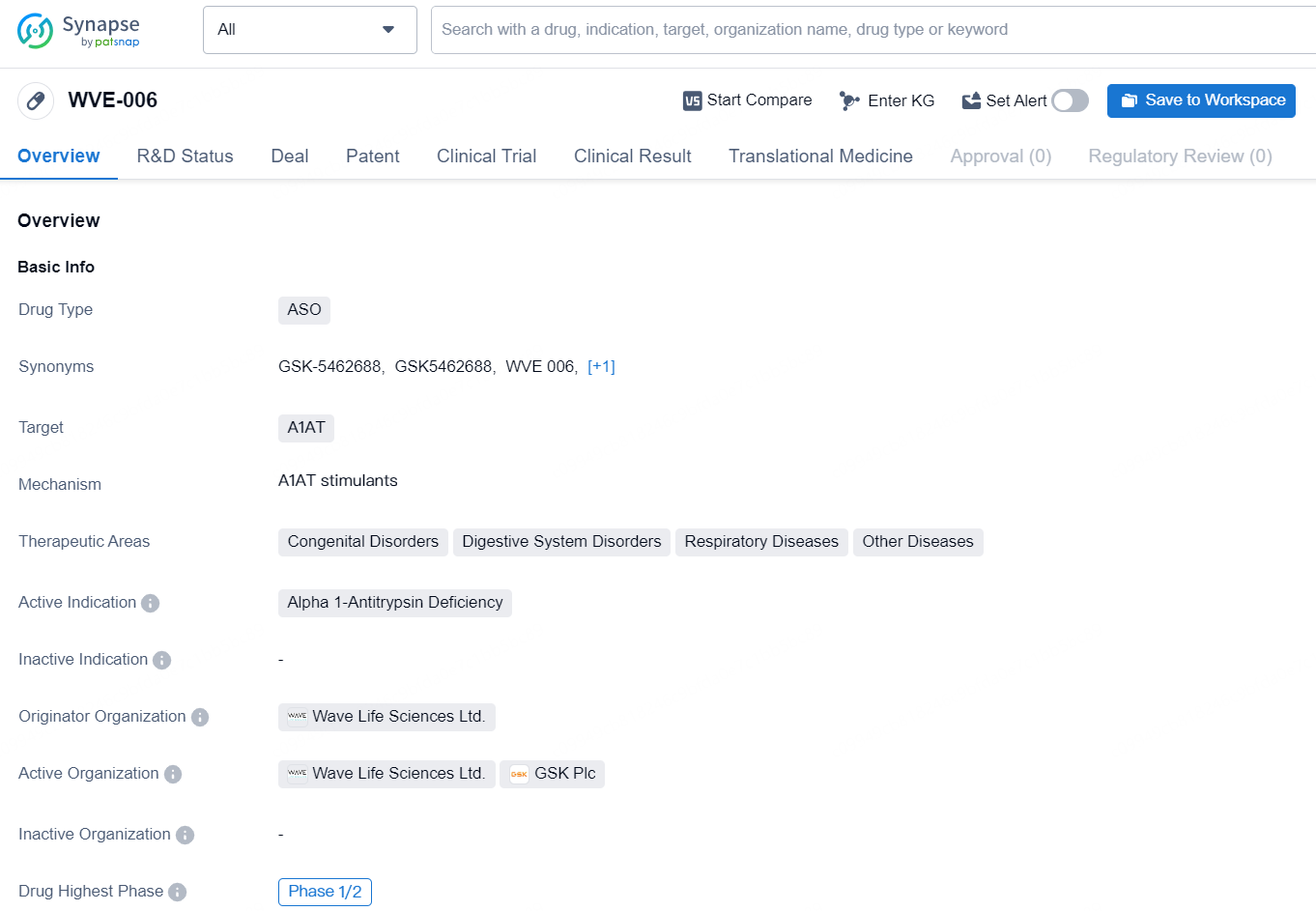
The drug WVE-006 is an antisense oligonucleotide (ASO) that targets Alpha-1 Antitrypsin (A1AT). It is being developed by Wave Life Sciences Ltd. for the treatment of Alpha 1-Antitrypsin Deficiency, which falls under the therapeutic areas of congenital disorders, digestive system disorders, respiratory diseases, and various other diseases.