What ADC-related progress will Mabwell (Shanghai) Bioscience reveal at the ASCO conference?
Mabwell (Shanghai) Bioscience is an innovative biopharmaceutical company with a full industrial chain layout that has developed multiple Antibody-Drug Conjugate (ADC) technology platforms. The company primarily focuses on oncology and age-related diseases, encompassing therapeutic areas such as cancer, autoimmune disorders, metabolism, ophthalmology, and infections. At the upcoming ASCO conference, the company will present the Phase I/II clinical study results of 9MW2821 for several advanced solid tumors through an oral report.
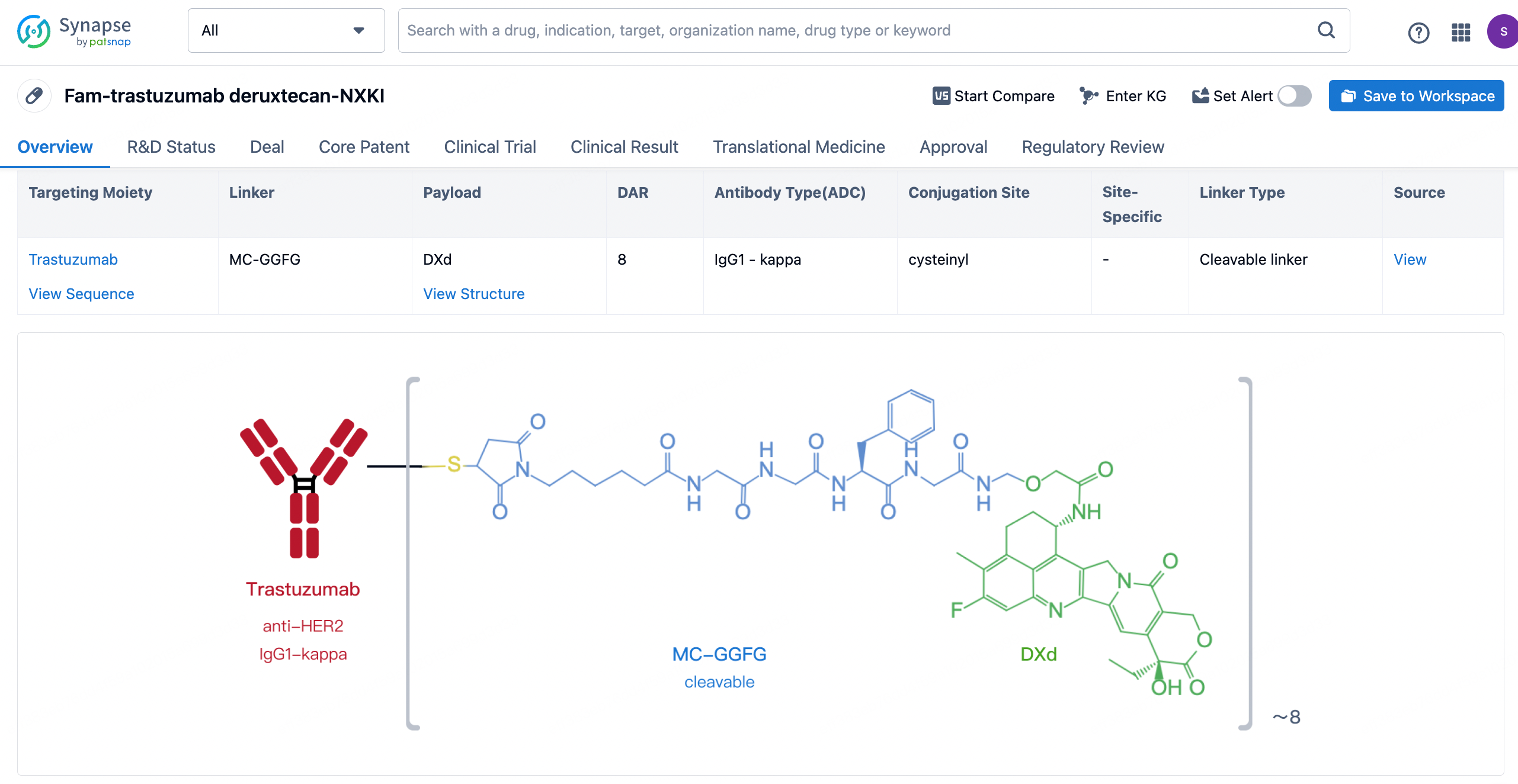
9MW2821 employs a site-specific linker-coupling technology developed in collaboration with the Shanghai Institute of Materia Medica, Chinese Academy of Science. This technology couples the MMAE toxin and, through optimizations in the coupling process, addresses common issues such as linker mismatches, avoiding problems like ADC aggregation for enhanced stability. The antibody is humanized and optimized for post-translational modifications (PTM), with a drug-to-antibody ratio (DAR) of 4.
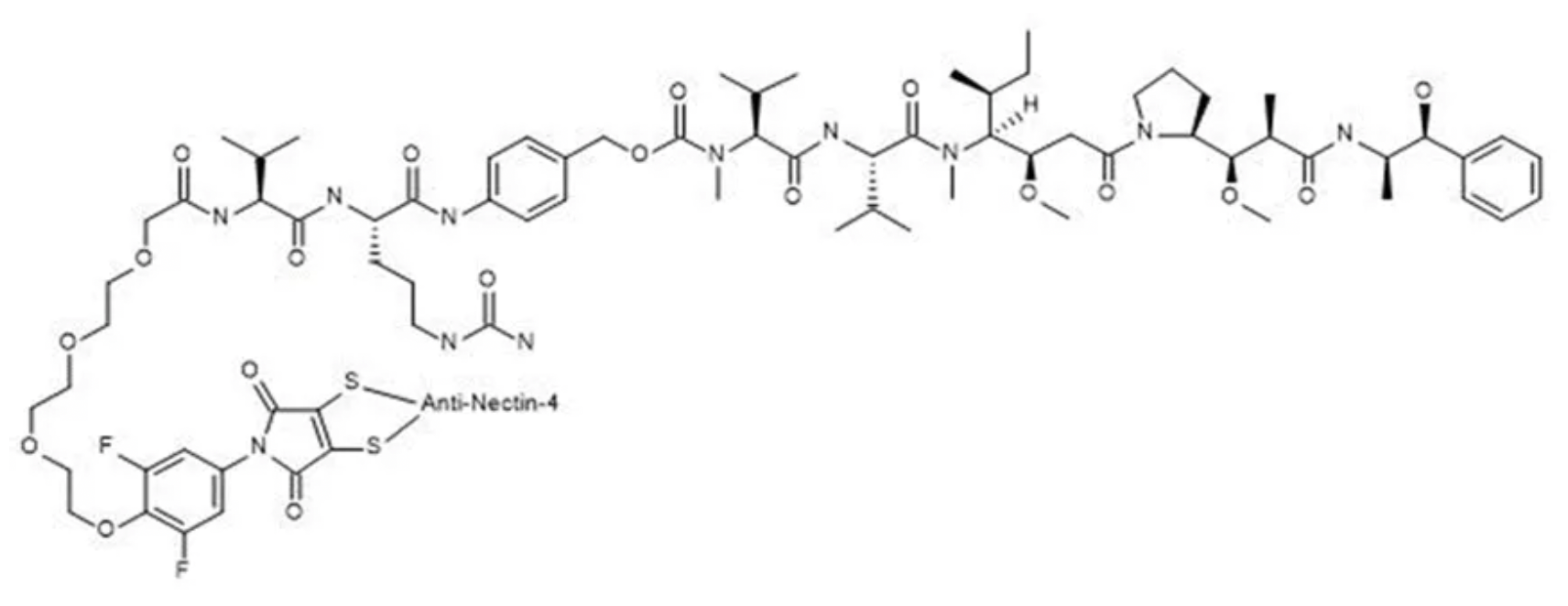
In February 2024, 9MW2821 was granted "Fast Track Designation" (FTD) by the FDA for treating advanced, recurrent, or metastatic esophageal squamous cell carcinoma. It is currently the first targeted therapy against Nectin-4 globally to reveal clinical efficacy and safety data for indications of esophageal and cervical cancer.
Currently, four clinical trials involving 9MW2821 have been initiated:
1.An open, multicenter Phase I clinical study to evaluate the safety, tolerability, pharmacokinetics, and preliminary efficacy of 9MW2821 in patients with advanced solid tumors, with plans to enroll 40 patients.
2.A Phase I/IIa clinical trial to assess the safety, tolerability, pharmacokinetics, and preliminary efficacy of 9MW2821 in patients with advanced solid tumors, aiming to enroll 208 patients.
3.An open, single-arm, multicenter Phase Ib/II clinical study of 9MW2821 combined with Junshi Biosciences' PD-1 monoclonal antibody, Toripalimab, evaluating safety and efficacy in patients with advanced or metastatic urothelial carcinoma, with plans to enroll 100 patients.
4.A randomized, open, controlled, multicenter Phase III clinical study comparing 9MW2821 in patients with locally advanced or metastatic urothelial carcinoma who have previously undergone chemotherapy that included platinum agents and PD-(L)1 inhibitors but are ineligible for surgical resection, planning to enroll 420 patients.
Urothelial Carcinoma (UC)
As of April 27, 2023, in 18 UC patients who progressed following treatment with platinum-based chemotherapy and immune checkpoint inhibitors, the dose of 1.25 mg/kg showed an Objective Response Rate (ORR) and Disease Control Rate (DCR) of 55.6% and 94.4%, respectively.
As of December 5, 2023, 9MW2821 at a dose of 1.25 mg/kg in a phase II clinical trial showed ORR and DCR of 62.2% and 91.9% respectively, in the monotherapy treatment of advanced urothelial carcinoma patients. The median Progression-Free Survival (PFS) was 6.7 months, and the median Overall Survival (OS) had not yet been reached.
Currently, the phase III clinical study 9MW2821-2023-CP301 of 9MW2821 monotherapy for locally advanced or metastatic urothelial carcinoma patients who have been previously treated with platinum-based chemotherapy and PD-(L)1 inhibitors has officially commenced. Furthermore, the phase I/II clinical study 9MW2821-2023-CP104 combining 9MW2821 with a PD-1 inhibitor is also progressing, with the first subject successfully enrolled.
Cervical Cancer (CC)
On March 16, 2024, MyWay Biotech reported positive clinical study results for their first cervical cancer study targeting Nectin-4 with an antibody-drug conjugate (ADC) at the 2024 SGO conference (First positive cervical cancer results for Nectin-4, MyWay announces clinical study 9MW2821).
The Phase I/II cervical cancer cohort of 9MW2821 included patients with Nectin-4 positive recurrent or metastatic cervical cancer, who have failed platinum-based doublet chemotherapy with or without bevacizumab (a VEGF monoclonal antibody), and have been treated with no more than two lines of systemic therapy.
As of September 25, 2023, within the expanded cervical cancer cohort of this study, the detection rate for Nectin-4 expression was 89.67%, with a 3+ tumor cell staining intensity detection rate of 67.82%. This cohort enrolled a total of 40 patients, with 57.5% of the participants previously treated with platinum-based doublet chemotherapy combined with bevacizumab, and 60% of participants previously having received platinum-based chemotherapy and immune checkpoint inhibitors.
In terms of efficacy, under the selected dosage of 1.25 mg/kg in Phase II, the objective response rate (ORR) and disease control rate (DCR) among 37 evaluable patients were 40.54% and 89.19% respectively, with 1 case of complete response (CR, 2.70%), 14 cases of partial response (PR, 37.84%), and median progression-free survival (mPFS), median overall survival (mOS), and median duration of response (mDOR) not yet reached. Among patients with Nectin-4 3+ expression, the ORR and DCR for 26 evaluable patients were 50.00% and 92.31% respectively. For patients previously treated with platinum-based chemotherapy and immune checkpoint inhibitors, the ORR and DCR among 21 evaluable patients were 38.10% and 85.71%, respectively.
Esophageal Squamous Cell Carcinoma (ESCC)
As of February 20, 2024, in the phase II clinical trial of 9MW2821 at a dosage of 1.25 mg/kg, among 30 patients with advanced esophageal cancer who received monotherapy and completed at least one tumor efficacy evaluation, the objective response rate (ORR) and disease control rate (DCR) were 30% (9/30) and 73.3% (22/30), respectively.
The phase II clinical study targeting esophageal cancer indications will continue to enroll and evaluate patients and will initiate phase III clinical discussions as soon as possible.
In February 2024, 9MW2821 was granted Fast Track Designation (FTD) by the FDA for the treatment of advanced, recurrent, or metastatic esophageal squamous cell carcinoma (This is the first Nectin-4 ADC showing positive results for ESCC; 9MW2821 received FTD from the FDA).
Safety
Clinical trial results for 9MW2821 targeting advanced solid tumors were orally presented at the 2023 ESMO Congress held in Madrid, Spain, from October 20-24, 2023. As of April 27, 2023, the study had enrolled 97 patients, including 39 with urothelial carcinoma and 29 with cervical cancer. The age range of the patients was 32-78 years (median age of 57 years), and the dosage ranged from 0.33 to 1.5 mg/kg. These subjects had previous treatments with platinum-based chemotherapy and immune checkpoint inhibitors.
There were no treatment-related deaths in the study. Only one case of dose-limiting toxicity was observed in the 1.5 mg/kg group, which was grade 4 neutropenia lasting more than 5 days. The maximum tolerated dose (MTD) has not yet been reached.
The incidence of any grade treatment-related adverse events (TRAEs) was 64.9%. The most common treatment-related adverse events were decreased white blood cell count (36.1%, 35/97), neutropenia (35.1%), nausea (22.7%), increased aspartate aminotransferase (22.7%), rash (19.6%), hair loss (19.6%), fatigue (18.6%), decreased appetite (18.6%), anemia (17.5%), vomiting (16.5%), and peripheral neuropathy (16.5%). The incidence of grade 3/4 TRAEs was 35.1%, with the most common being decreased white blood cell count (18.6%) and neutropenia (18.6%).
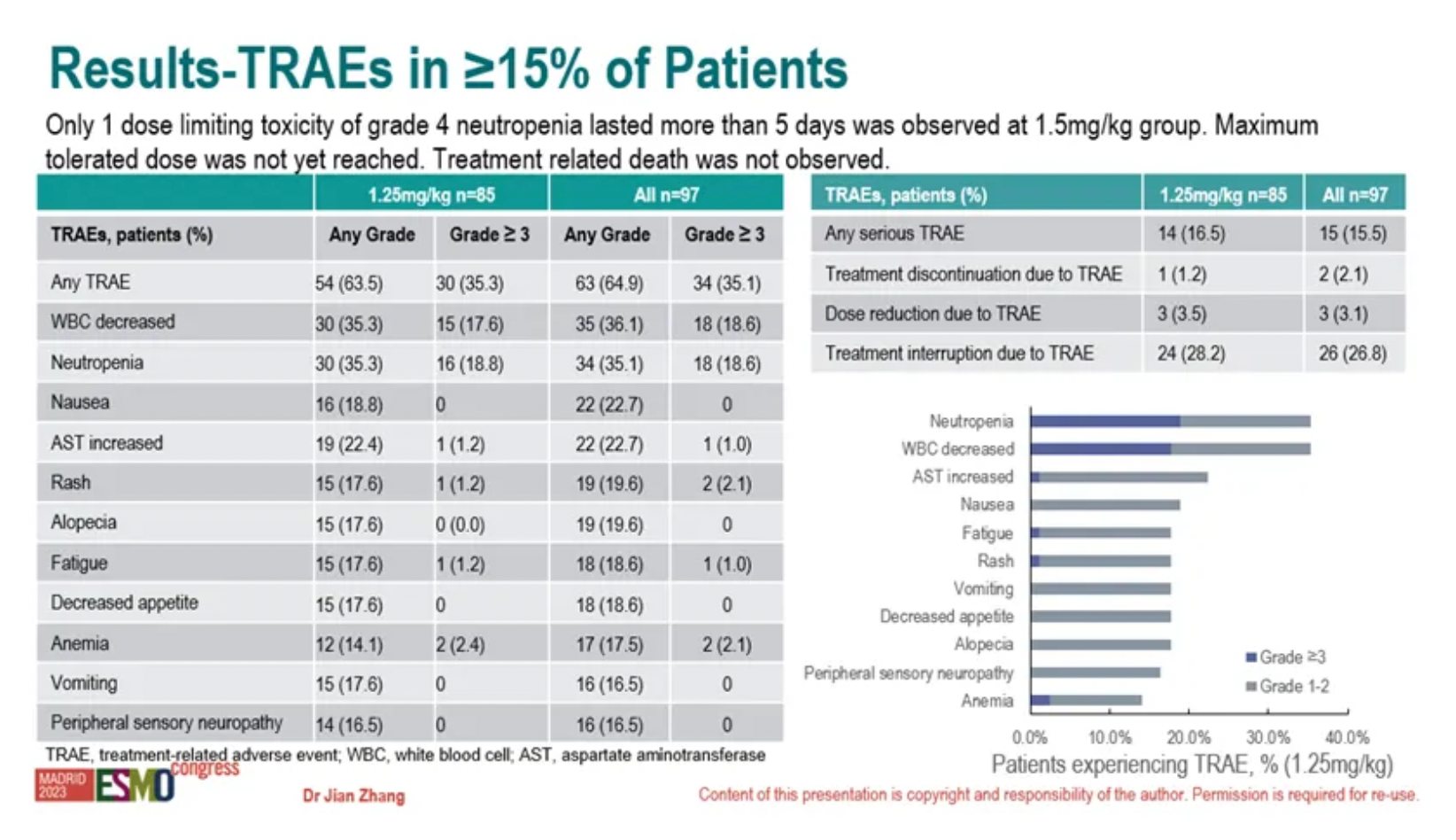
On March 16, 2024, Mabwell (Shanghai) Bioscience announced at the Society of Gynecologic Oncology (SGO) Annual Meeting through a Focused Plenary Oral Presentation the positive clinical trial results of its Nectin-4 targeted ADC in cervical cancer.
As of September 25, 2023, 92.50% (37/40) of the participants experienced treatment-related adverse events, with the most common grade 3 treatment-related adverse events being neutropenia at 40.00% (16/40), rash at 17.50% (7/40) and increased gamma-glutamyl transferase at 12.50% (5/40). There were no treatment-related deaths reported.
Mabwell's ADC Pipeline
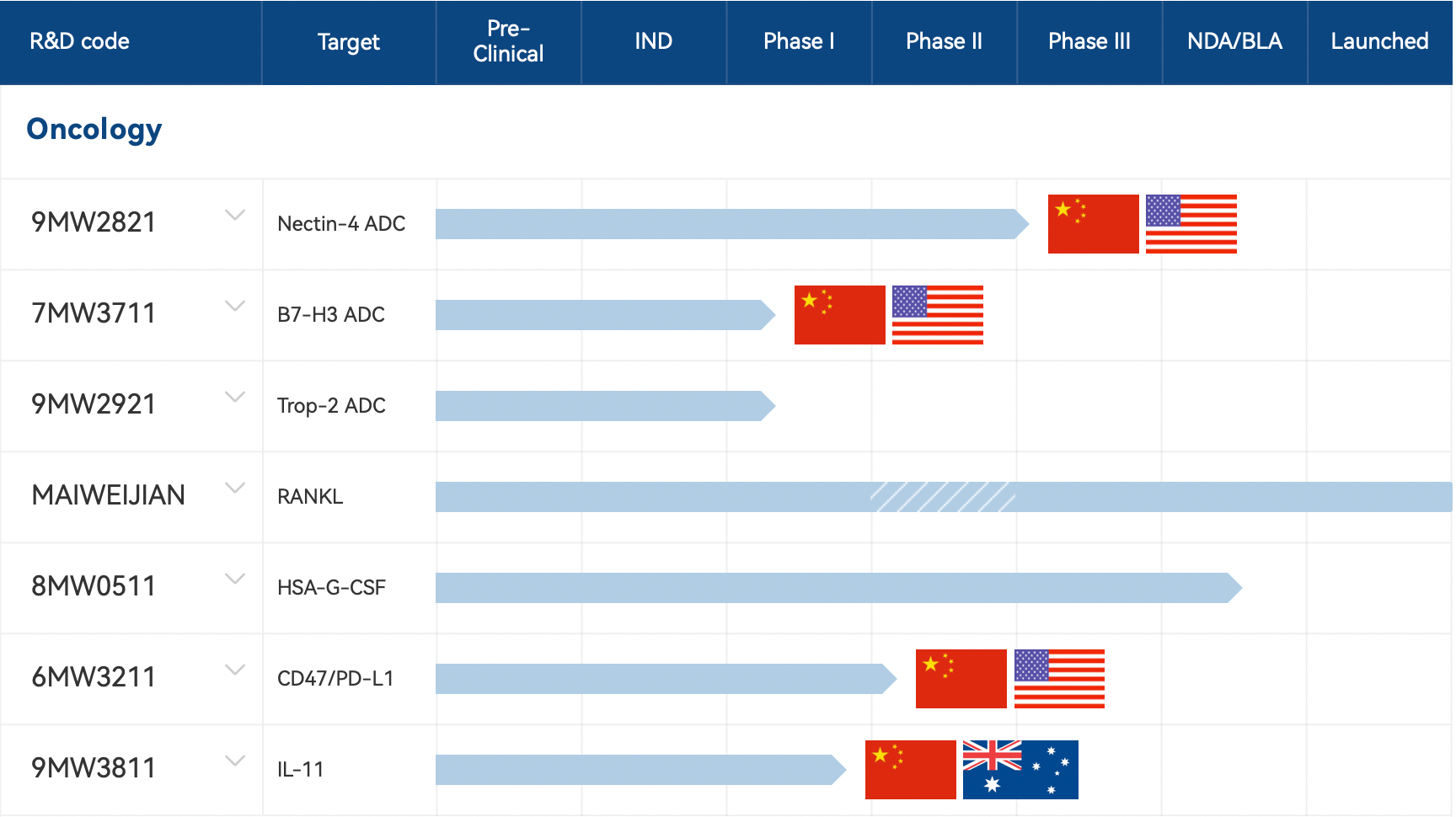
Mabwell (Shanghai) Bioscience has developed two antibody-drug conjugates (ADCs), B7H3 ADC 7MW3711 and TROP2 ADC 9MW2921, based on its next-generation conjugation technology platform IDDC™. Both ADCs are currently in Phase I clinical trials.
IDDC™ is a next-generation site-specific conjugation technology platform autonomously developed by Mabwell Bioscience, comprising of several systematic core patented technologies: the site-specific conjugation process DARfinity™, the site-specific linker IDconnect™, the novel payload molecule Mtoxin™, and the conditional release structure LysOnly™. The next-generation ADCs developed using these systematic patented technologies exhibit improved structural uniformity, quality stability, efficacy, and tolerance.
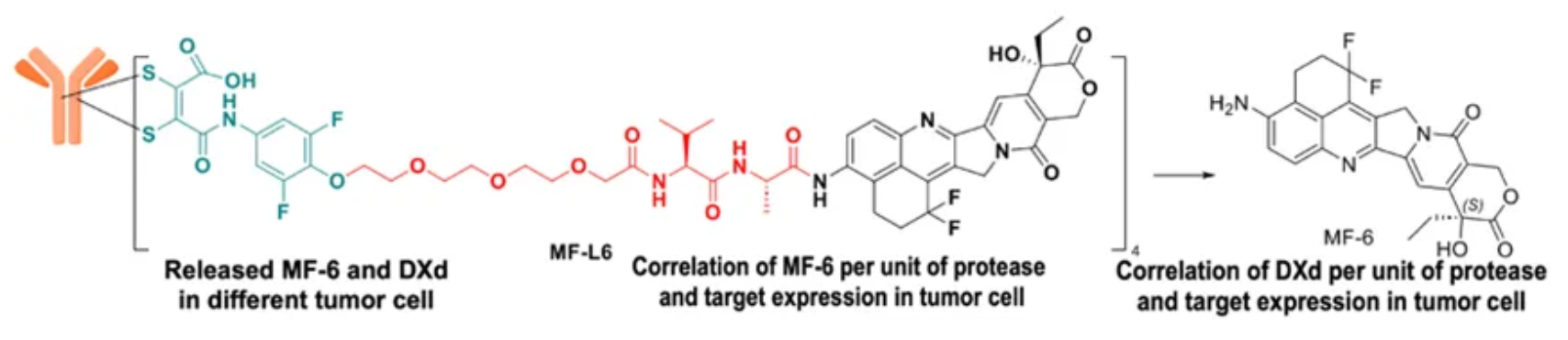
The LysOnly™ technology enhances the tumor-specific release capacity of ADC drugs, reducing off-target effects.
How to search for and analyze the development progress of ADC pharmaceuticals?
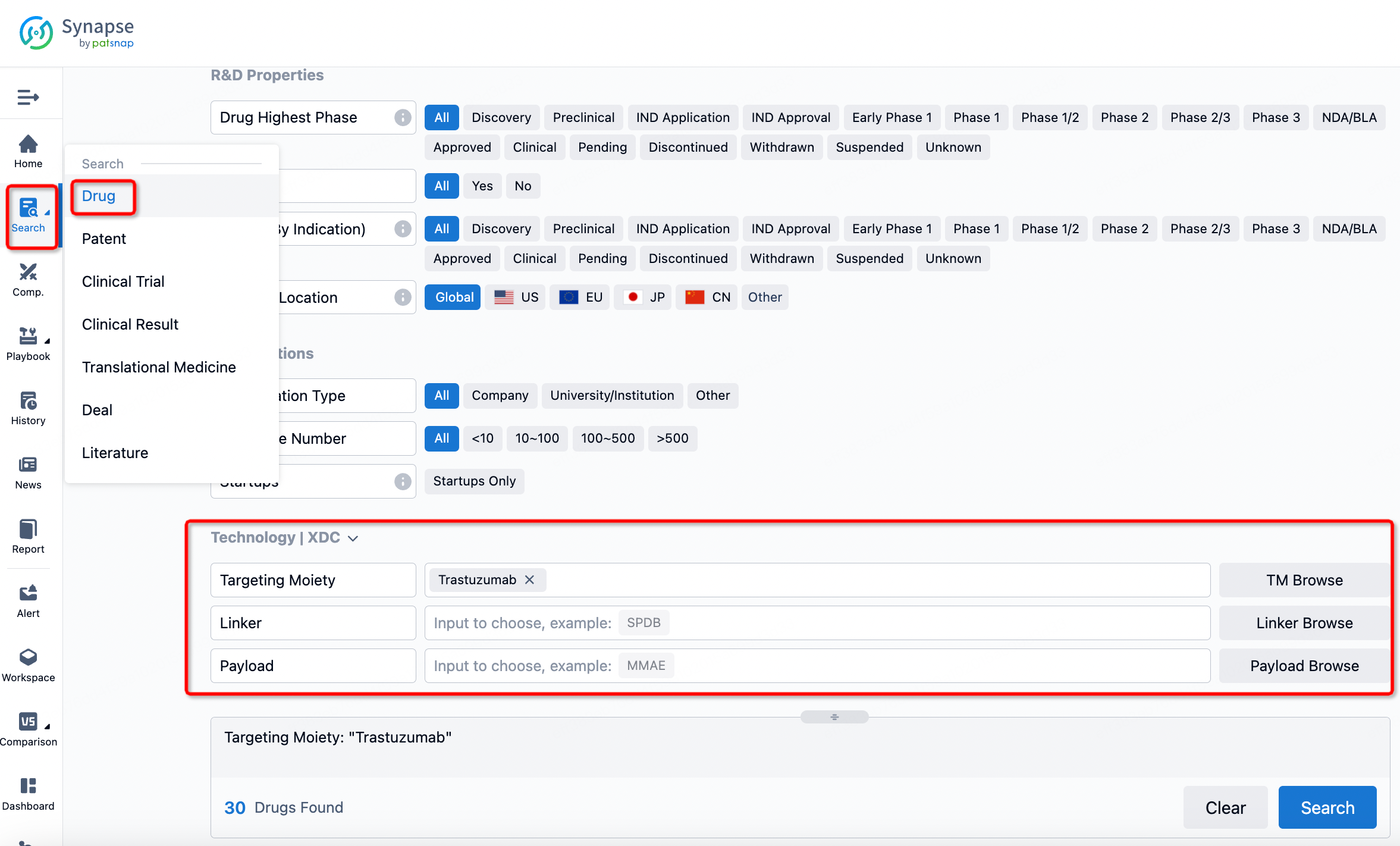
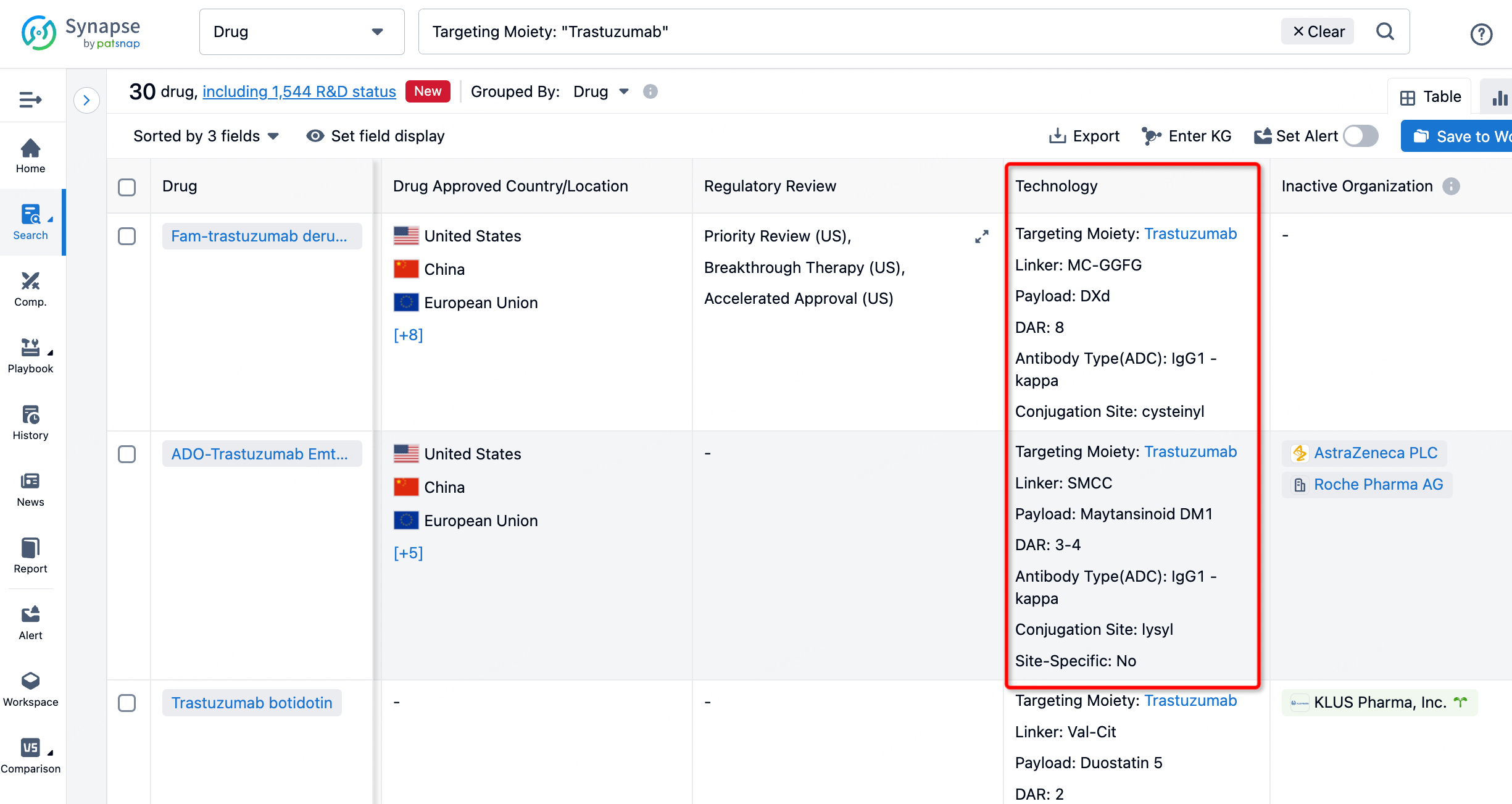
If you want to learn about the latest developments in ADC drugs, you can use the drug search module of the Synapse database. This module supports searching for ADC drugs by classification through Targeting Moiety, Linker, and Payload.
On the search results page, you can easily review information related to the ADC's technical category of the drugs.
After clicking to enter the drug details page, you can also effortlessly obtain structural information about the ADC drug.
Click on the image below to embark on a brand new journey of drug discovery!