What are TLR7 agonists and how do you quickly get the latest development progress?
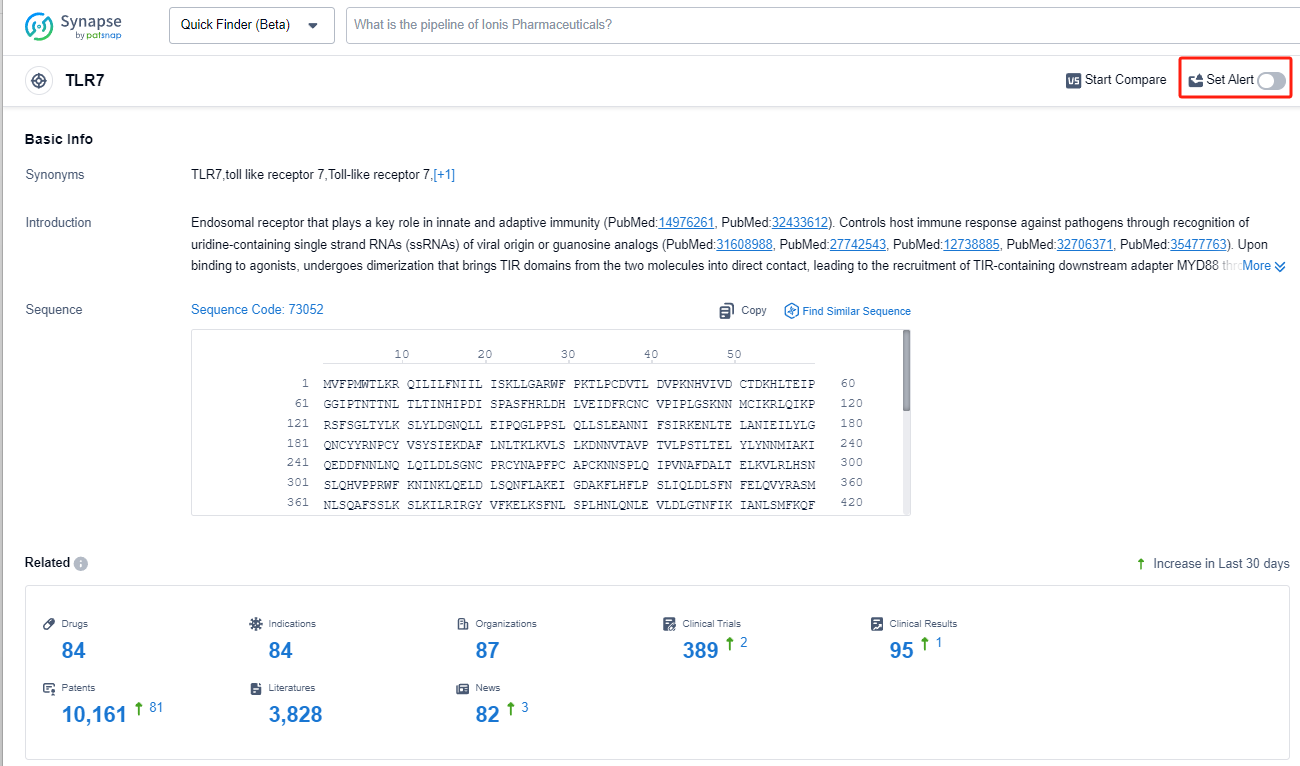
TLR7, or Toll-like receptor 7, is a crucial component of the innate immune system in humans. It is a pattern recognition receptor that recognizes viral RNA and triggers an immune response against viral infections. TLR7 is primarily expressed in immune cells such as dendritic cells and macrophages. Upon activation, TLR7 initiates a signaling cascade that leads to the production of pro-inflammatory cytokines and type I interferons, which are essential for antiviral defense. Understanding the role of TLR7 in the human body is vital for developing therapeutic interventions targeting viral infections and autoimmune diseases.
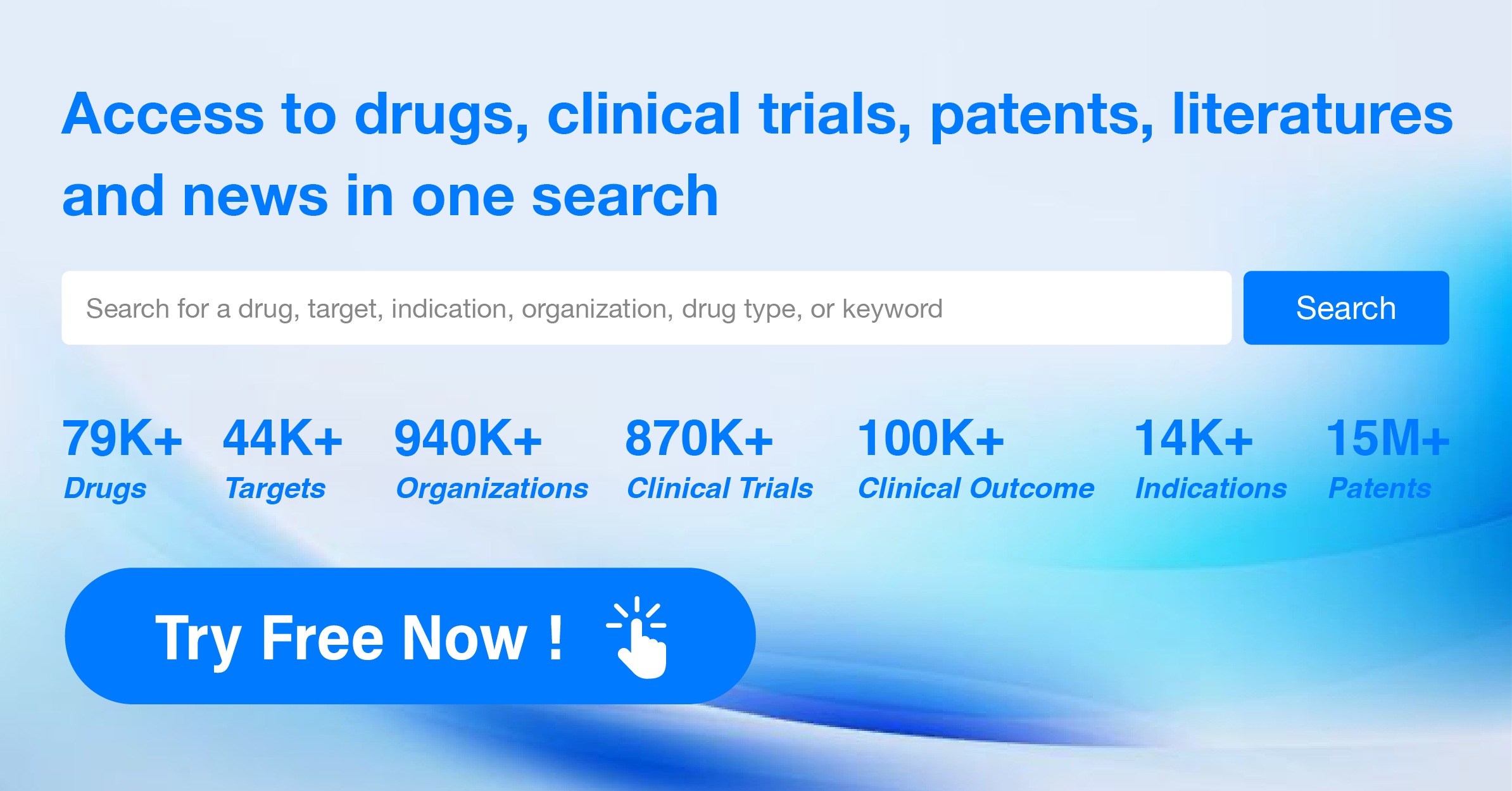
The analysis of target TLR7 from various perspectives provides valuable insights into the current competitive landscape and future development. Companies such as Roche Holding AG, Viatris Inc., and Bausch Health Cos., Inc. have shown significant progress in the development of drugs targeting TLR7. Indications such as hepatitis B, various types of cancer, and infectious diseases have received attention in the development of drugs under target TLR7. The presence of small molecule drugs and monoclonal antibodies indicates intense competition, while the involvement of biosimilar research and development institutions should be monitored. The United States, European Union, and China are leading in terms of research and development activities for target TLR7. Overall, the analysis suggests a promising future for the development of drugs targeting TLR7, with potential breakthroughs in the treatment of various diseases.
How do they work?
TLR7 agonists are a type of compounds that activate Toll-like receptor 7 (TLR7) in the immune system. Toll-like receptors are proteins that play a crucial role in recognizing and responding to pathogens, such as viruses and bacteria. TLR7 specifically recognizes viral RNA, triggering an immune response to eliminate the invading virus.
TLR7 agonists are designed to mimic viral RNA and activate TLR7, thereby stimulating the immune system to mount a robust antiviral response. They can enhance the production of cytokines, such as interferons and pro-inflammatory molecules, which help in the elimination of viral infections. Additionally, TLR7 agonists can promote the maturation and activation of immune cells, such as dendritic cells, which are essential for initiating and coordinating immune responses.
From a biomedical perspective, TLR7 agonists have gained attention as potential therapeutic agents for viral infections, including respiratory viruses like influenza and SARS-CoV-2 (the virus causing COVID-19). By activating TLR7, these agonists can boost the immune response against the virus, potentially reducing viral replication and improving clinical outcomes.
It is important to note that TLR7 agonists are still under investigation and development, and their safety and efficacy need to be thoroughly evaluated through preclinical and clinical studies before they can be used as approved treatments.
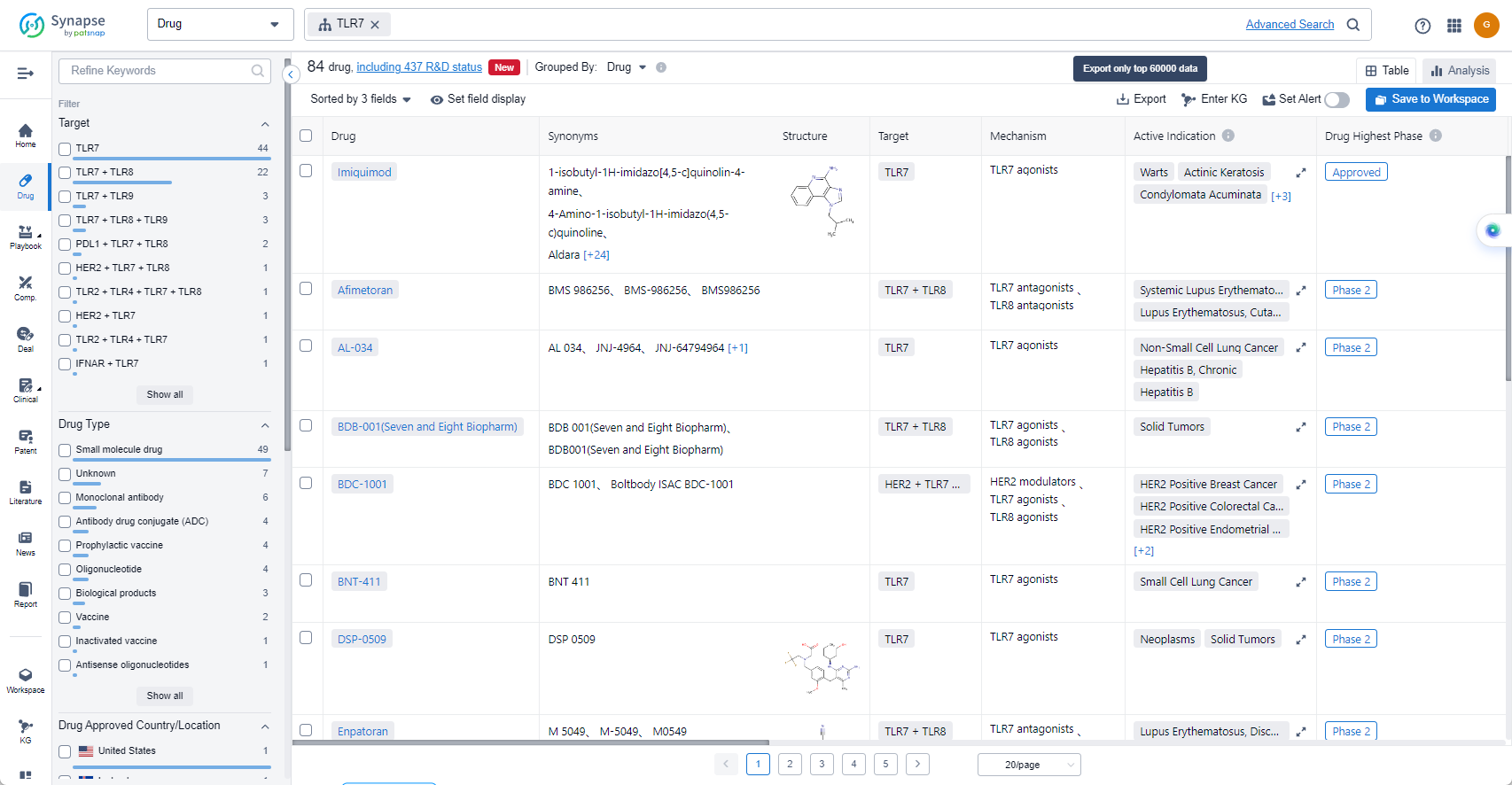
List of TLR7 Agonists
The currently marketed TLR7 agonists include:
- Imiquimod
- Afimetoran
- AL-034
- BDB-001(Seven and Eight Biopharm)
- BDC-1001
- BNT-411
- DSP-0509
- Enpatoran
- NKTR-262
- Resiquimod
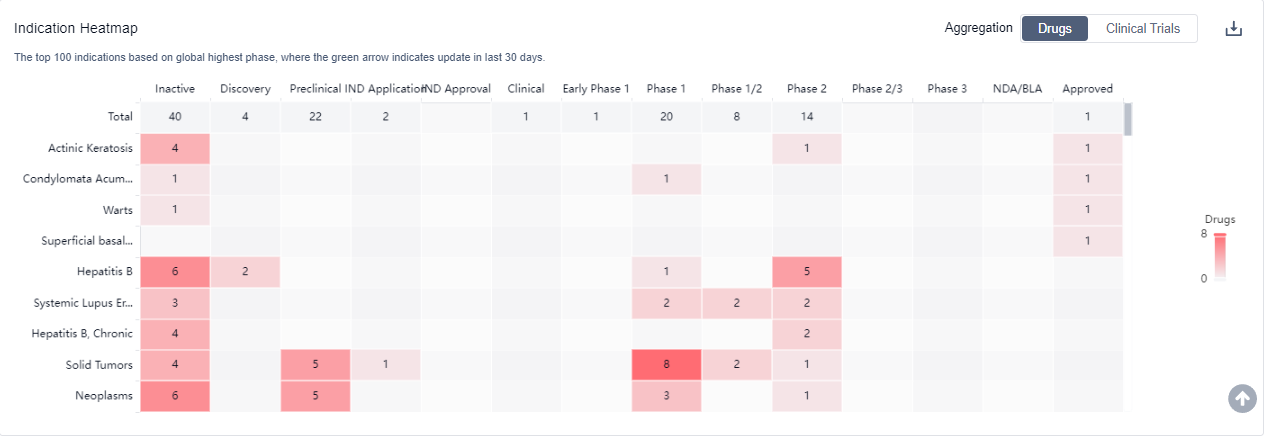
For more information, please click on the image below.
What are TLR7 agonists used for?
TLR7 agonists are primarily being studied for their role in immunotherapy, specifically in oncological indications. For more information, please click on the image below to log in and search.
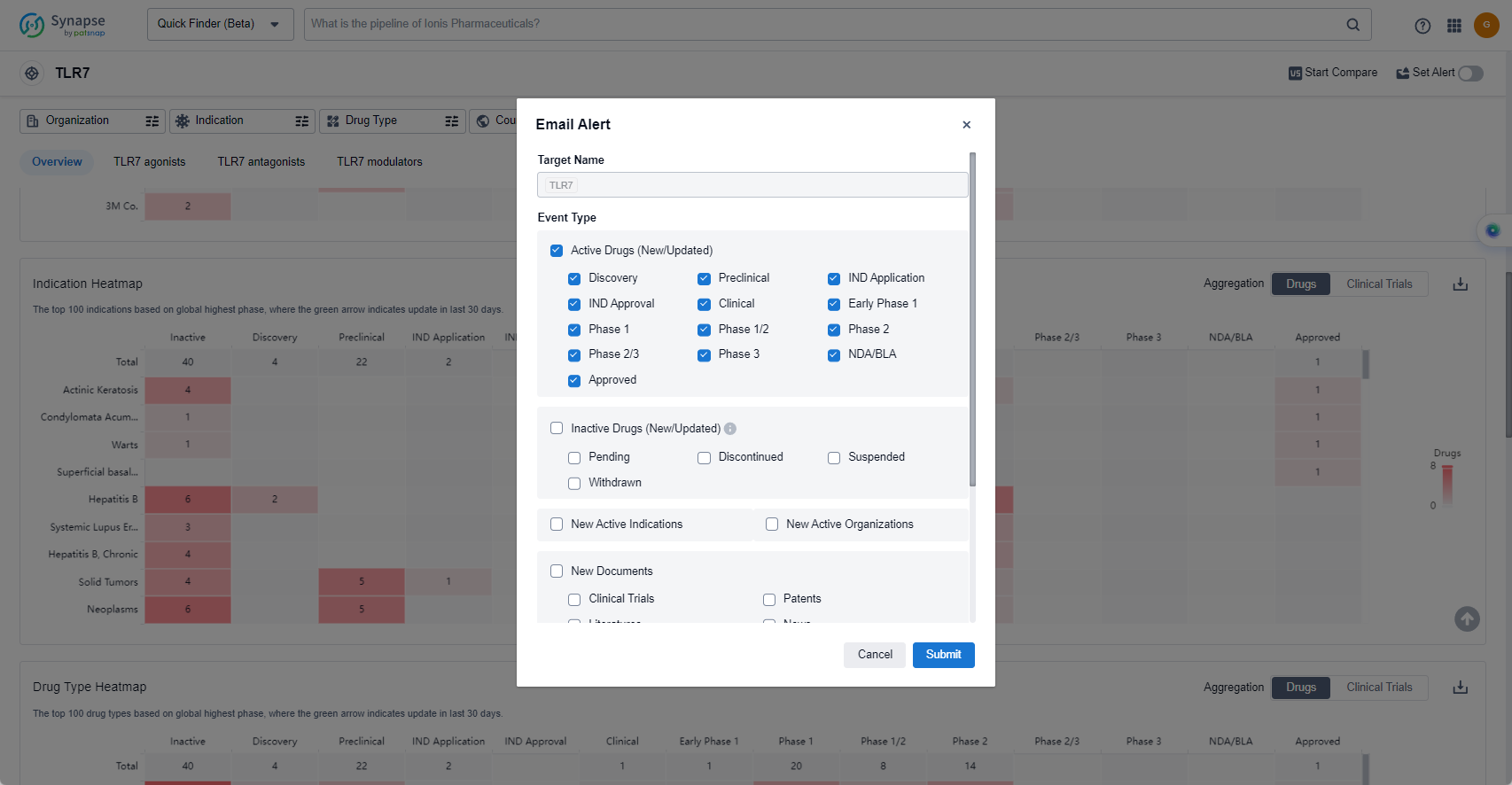
How to obtain the latest development progress of TLR7 agonists?
In the Synapse database, you can keep abreast of the latest research and development advances of TLR7 agonists anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!