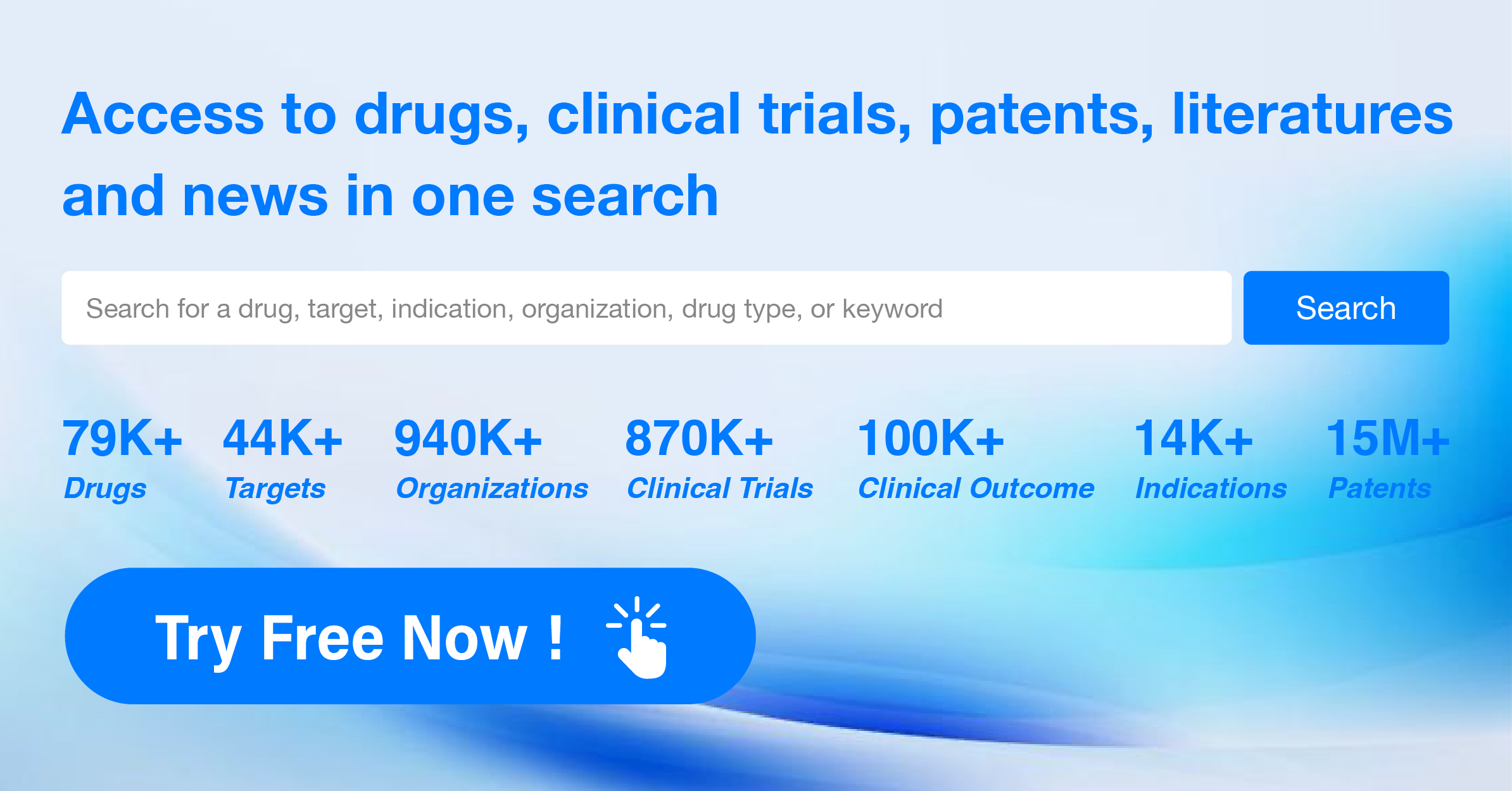
What is CBER's role after a biological product is approved and in use?
CBER continues to monitor the safety and stability of biological products that have been approved. Manufacturers must report certain problems to FDA's Biological Product Deviation Reporting System. Manufacturers must also report and correct product problems within established timeframes. If a significant problem is detected, a manufacturer may need to recall a product or even stop manufacturing it.
CBER encourages health professionals and members of the public to report problems with biological products to FDA's MedWatch and, for vaccines, to the Vaccine Adverse Event Reporting System (VAERS).
CBER inspects manufacturing plants before it approves products, and thereafter, on a regular basis. The purpose of these inspections is to assess whether biological products are made in compliance with appropriate laws and regulations, and to assist in identifying any changes needed to help ensure product quality.
CBER also may inspect clinical study sites to determine whether the studies are being carried out properly, and to ensure that accurate information is being submitted to the agency. CBER has a number of enforcement tools that may be used when a manufacturer or clinical researcher is in violation of FDA laws and regulations.