NRx Pharmaceuticals (Nasdaq:NRXP) Reports First Quarter 2024 Financial Results and Provides Business Update
14 May 2024
Financial StatementFast TrackNDAClinical ResultPriority Review
2024 Catalysts: Positive Clinical Data, Two Planned NDAs, Company Launch of Hope Therapeutics; FDA QIDP award in cUTI and New Schizophrenia Opportunity
Executed Term Sheet from an institutional investor for an initial $7.5 million note, subject to common closing requirements, primarily to replace current debt, clearing the path to a Hope Therapeutics share distribution, with provision for funding up to $30 million to fund pipeline opportunities
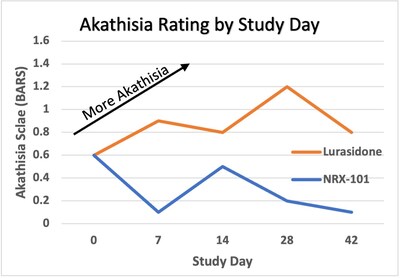
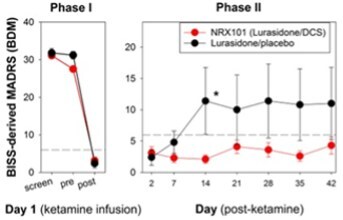
Positive data from a Phase 2b/3 trial of NRX-101 in Treatment Resistant Bipolar Depression (TRBD); trial demonstrated depression efficacy comparable to standard of care and significant reduction of akathisia (P=0.025). Akathisia is a potentially lethal side effect of all serotonin-targeted antidepressants and is associated with suicide. The study additionally demonstrated a 30% advantage in sustained remission from suicidality that was not statistically-significant at this sample size
Above findings of reduced suicidality mirror the results of the Company's prior STABIL-B trial in acutely suicidal patients and also mirror the results of an independent published trial
Company plans to file a New Drug Application (NDA) for Accelerated Approval under Breakthrough Therapy and Priority Review of NRX-101 in treatment of bipolar depression in people at risk of akathisia, based on the Phase 2b/3 and STABIL-B data
Company has developed patentable pH neutral formulation for ketamine that will be suitable for both intravenous and subcutaneous administration. Ketamine efficacy data are in hand from 4 clinical trials. Three manufacturing lots are now initiated (required for NDA) and Company plans to initiate the NDA by July
HOPE Therapeutics (which focuses on care delivery, not drug development) has partnered with representatives of ketamine clinic providers nationwide to construct a care platform that will include ketamine, operational support, and digital therapeutic extensions. In advance of FDA approval, HOPE is actively in the sales process to supply ketamine under 503b pharmacy licensure to meet the national ketamine shortage declared by FDA. HOPE is planned to be spun out as a separate company to be owned by NRx, current NRx shareholders, and new investors; Term Sheets received from prospective anchor investors for $60 million of new investment, once publicly listed
Data expected shortly in 200-person DOD-funded trial of D-cycloserine (DCS), the key component of NRX-101, to treat chronic pain, conducted by Northwestern University. Statistical analysis plan and data unlock have been approved by Northwestern IRB
NRX-101 in the treatment of Complicated Urinary Tract Infection (cUTI) granted Qualified Infectious Disease Product (QIDP), Fast Track, and Priority Review designations. Company has now demonstrated that NRX-101 does not damage the microbiome of the gut, in contrast to all other advanced antibiotics and is less likely to cause C. Difficile infection (a potentially lethal side effect of antibiotic treatment). NRx is reviewing partnership options
Executed Memorandum of Understanding with Fondation FundaMental for rights to develop potential disease modifying drug for Schizophrenia. If successful, this would represent the first drug to reverse the underlying disease mechanism of schizophrenia, rather than simply treating symptoms.
Management to host a conference call, May 14, 2024, at 4:30 PM ET
RADNOR, Pa., May 14, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced its financial results for the quarter ended March 31, 2024, and provided a business update.
Preview
Source: PRNewswire
Akathisia rating by study day: a consistent effect is seen commencing at first post-randomization visit and continued throughout the study (Mixed Model for Repeated Measures Regression effect size =0.037, P=0.025)
Preview
Source: PRNewswire
STABIL-B

Preview
Source: PRNewswire
HOPE Therapeutics, Inc. (PRNewsfoto/NRx Pharmaceuticals, Inc.)
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Indications
Targets
-Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.





