Pheburane® (sodium phenylbutyrate) is now on the Medicaid Preferred Drug List in 10 states
23 Jan 2024
Drug Approval
PRINCETON, NJ, Jan. 23, 2024 /PRNewswire/ -
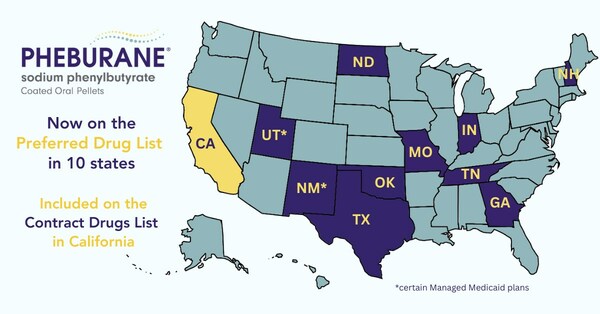
Medunik USA, member of Duchesnay Pharmaceutical Group (DPG) one of the few anchor companies selected by the Government of Canada for its Global Hypergrowth Project, is pleased to announce that Pheburane® coverage has reached another important milestone to the benefit of Americans with certain urea cycle disorders (UCDs) and is now on the Medicaid Preferred Drug List in 10 states with the recent addition of Georgia, Indiana, Tennessee, and Missouri along with those already in effect in New Hampshire, New Mexico*, North Dakota, Oklahoma, Texas, and Utah*. Pheburane® is also included on the California Contract Drugs List with no prior authorization required and is covered by commercial insurance plans, which now reaches ~80% of the commercially insured US population.
Continue Reading
Preview
Source: PRNewswire
Pheburane® (sodium phenylbutyrate) is now on the Medicaid Preferred Drug List in 10 states (CNW Group/Medunik USA)
Pheburane® is the safe, effective and palatable adjunctive therapy to the standard of care, which includes dietary management, for the treatment of certain UCDs. Pheburane® is an innovative formulation of sodium phenylbutyrate consisting of very small coated oral pellets (about the size of sugar crystals) and is indicated for both adult and pediatric patients. Pheburane® is not indicated for the treatment of acute hyperammonemia. The most common side effects associated with sodium phenylbutyrate are menstrual dysfunction, decreased appetite, body odor and bad taste or taste aversion.1
Available in Europe since 2013 and in Canada since 2015, Pheburane® has demonstrated globally its effectiveness in the treatment of UCDs. Pheburane® is a proven treatment with both short-term and long-term (up to 30 months) clinical data establishing its treatment efficacy at lowering ammonia levels in the blood to within normal range2,3 which is crucial as high ammonia levels in the bloodstream may lead to serious and life-threatening health problems for UCD patients.4
"We are glad to contribute to Medunik USA's efforts to facilitate availability of Pheburane® for Americans with certain urea cycle disorders. The widespread access we have been able to achieve reflects the value proposition of Pheburane® to the healthcare system, and we look forward to continuing to assist Medunik USA in their efforts to generate further cost-savings throughout the healthcare industry," said Bill Finneran, President of Viking Healthcare Solutions.
With the continued favorable commercial and Medicaid coverage updates, Pheburane® is reaching more patients all over the USA while being recognized as a first-line therapy by health authorities in key states. In other states Pheburane® has equal, or better, coverage as well as being priced at a significant discount to these options: 1/3 of the cost of RAVICTI® (glycerol phenylbutyrate)** and ½ the price of OLPRUVATM (sodium phenylbutyrate)**.
UCDs are rare, chronic, genetic conditions that can be fatal if left untreated, and can impact children from the time of birth. UCDs disrupt the body's urea cycle, and therefore, the body is unable to remove the dangerous buildup of toxic chemicals, particularly ammonia, that are created from the digestion of protein. One in 35,000 people in the United States or about 28 per one million residents suffer from UCDs of different levels of severity.4
Medunik USA offers Pheburane® through its UNIK Support Program – designed to support each unique patient. UNIK Support offers specialized services including a copay savings program, patient care liaison services, mail order pharmacy and other support services. Visit Pheburane.com for more information.
*Certain Managed Medicaid plans
**Based on published pricing information.
All trademarks are the property of their respective owners.
The main purpose of the Urea Cycle is to eliminate toxic ammonia from the blood and make urea, which is then excreted as urine. UCDs are rare genetic disorders that cause errors in this process, allowing high levels of ammonia, the key marker for UCDs, to build up in the bloodstream, potentially to dangerous and fatal levels. Ammonia is extremely toxic, particularly to the central nervous system. UCDs can cause catastrophic illness in newborns within 36 to 48 hours of birth despite the infants appearing normal, so they can be discharged from hospital before signs of UCDs develop. UCDs require lifelong monitoring and treatment.4
About Pheburane®
Pheburane® is a taste-masked oral formulation of sodium phenylbutyrate, approved by the Food and Drug Administration as adjunctive therapy to standard of care, which includes dietary management, for the chronic management of adult and pediatric patients with UCDs, involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC) or argininosuccinic acid synthetase (AS). Pheburane® is not indicated for the treatment of acute hyperammonemia.1
Pheburane®, developed by Lucane Pharma, is under exclusive distribution in the U.S. through Medunik USA. For further information, visit Pheburane.com.
INDICATION AND IMPORTANT SAFETY INFORMATION
What is Pheburane®?
Pheburane® is a prescription medicine, used along with a specific diet, for the long-term management of adults and children with urea cycle disorders (UCDs), involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC) or argininosuccinate synthetase (AS).
Episodes of sudden, rapid increase of ammonia in the blood (acute hyperammonemia) may happen in people during treatment with
Pheburane® is not used for the treatment of acute hyperammonemia, which can be life-threatening and requires emergency medical treatment.
Before
taking Pheburane®, tell your healthcare provider about all of your medical conditions, including if you:
have heart problems.
have kidney or liver problems.
have diabetes (
Pheburane® contains sucrose), or have a history of fructose intolerance, glucose-galactose malabsorption, or sucrose-isomaltase insufficiency.
are pregnant or plan to become pregnant. It is not known if
Pheburane® will harm your unborn baby.
are breastfeeding or plan to breastfeed. It is not known if
Pheburane® passes into your breastmilk. Talk to your healthcare provider about the best way to feed your baby during treatment with
Tell your healthcare provider about all the medicines you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Certain medicines may increase the level of ammonia in your blood or cause serious side effects when taken during treatment with Pheburane®. Especially tell your healthcare provider if you or your child take:
corticosteroids
haloperidol
Know the medicines you take. Keep a list of them to show your or your child's healthcare provider and pharmacist when you get a new medicine.
What are the possible side effects of Pheburane®?
can cause serious side effects, including:
Nervous system problems (neurotoxicity). Call your healthcare provider right away if you get any of the following symptoms during treatment with
Low potassium levels in your blood (hypokalemia). Your healthcare provider will monitor your blood potassium levels during treatment with PHEBURANE and treat if needed.
Conditions related to swelling (edema).
Pheburane® contains salt (sodium), which can cause swelling from salt and water retention. Your healthcare provider will decide if PHEBURANE is right for you if you have certain medical conditions that can cause swelling, such as heart failure, liver problems or kidney problems.
The most common side effects of Pheburane® include:
Your healthcare provider may do certain blood tests to check you or your child for side effects during treatment with
These are not all the possible side effects of
Call your doctor for medical advice about side effects.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Please read the Full Prescribing Information and Patient Information at Pheburane.com.
About Medunik USA
Based in Princeton, New Jersey, Medunik USA is part of Duchesnay Pharmaceutical Group and works to improve the health and quality of life of Americans living with rare diseases by making orphan drug therapies available in the United States. Through its strategic partnerships, Medunik USA develops and provides Americans suffering from rare disease with access to orphan drugs that are not currently available in the U.S. Medunik USA makes critical medications to treat rare diseases available to American patients who might not otherwise have access to these medications. For more information, visit www.medunikusa.com.
About Duchesnay Pharmaceutical Group
Duchesnay Pharmaceutical Group (DPG), with its affiliated companies, is headquartered in Blainville, Quebec. The group consists of five pharmaceutical companies to meet the needs of patients in Canada, the United States, and abroad. The companies are Duchesnay and Duchesnay USA, both dedicated to women's health; Médunik Canada and Medunik USA, which provide treatments for rare diseases; and Analog Pharma, an American generic drugs company, specializing in authorized generics and orphan drugs. From its state-of-the-art manufacturing plant, DPG exports its innovative treatments to more than 50 countries. DPG is one of the 8 companies across the country chosen to participate in the Government of Canada's Global Hypergrowth Project. This appointment offers exclusive and personalized support for at least 2 years, in order to accelerate our growth to become an anchor firm in the Canadian economy.
The Group, through its proprietary research and development, and through exclusive partnerships, offers innovative treatments for a variety of medical conditions in women's health, urology, oncology as well as for rare diseases. DPG recognizes the dedication and professionalism of its employees and promotes a positive culture and flexible work environment. It is deeply committed to environmental responsibility and to giving back to the community through the support of various charitable organizations. For more information, please visit: https://duchesnaypharmaceuticalgroup.com/en
About Viking
Viking Healthcare Solutions has provided market access and commercialization services to the pharma and biotech industries for over 25 years, supporting traditional and rare/specialty products throughout their lifecycle. Our breadth and depth of access, account knowledge, and payer insights empower us to connect manufacturers and payers to drive access to medications for patients in need.
References
Pheburane® (sodium phenylbutyrate) oral pellets [Prescribing Information]. Medunik USA, Inc.
Kibleur Y, Dobbelaere D, Barth M, Brassier A, Guffon N. Results from a Nationwide Cohort Temporary Utilization Authorization (ATU) survey of patients in France treated with Pheburane® (Sodium Phenylbutyrate) taste-masked granules. Paediatr Drugs. 2014. https://pubmed.ncbi.nlm.nih.gov/24962711/
Kibleur Y, Guffon N. Long-Term Follow-Up on a Cohort Temporary Utilization Authorization (ATU) Survey of Patients Treated with Pheburane (Sodium Phenylbutyrate) Taste-Masked Granules. Paediatr Drugs. 2016 Apr;18(2):139-44. doi: 10.1007/s40272-015-0159-8. PMID: 26747635. https://pubmed.ncbi.nlm.nih.gov/26747635/
Cleveland Clinic, Urea Cycle Disorder, https://my.clevelandclinic.org/health/diseases/23470-urea-cycle-disorder
SOURCE Medunik USA
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.




