Request Demo
Last update 09 Dec 2025

Nagoya University
Last update 09 Dec 2025
Overview
Tags
Neoplasms
Nervous System Diseases
Other Diseases
Small molecule drug
Cell therapy
CAR-T
Disease domain score
A glimpse into the focused therapeutic areas
No Data
Technology Platform
Most used technologies in drug development
No Data
Targets
Most frequently developed targets
No Data
| Top 5 Drug Type | Count |
|---|---|
| Small molecule drug | 9 |
| CAR-T | 3 |
| Cell therapy | 2 |
| Chemical drugs | 1 |
| ASO | 1 |
Related
24
Drugs associated with Nagoya UniversityTarget |
Mechanism VEGF-A inhibitors [+1] |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc. United States |
First Approval Date26 Feb 2004 |
Target- |
Mechanism Cell replacements |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication |
Drug Highest PhasePhase 3 |
First Approval Ctry. / Loc.- |
First Approval Date- |
Target |
Mechanism NEK2 inhibitors |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication- |
Drug Highest PhasePhase 1 |
First Approval Ctry. / Loc.- |
First Approval Date- |
393
Clinical Trials associated with Nagoya UniversityNCT06983210
Multi-center, Open-Label, Single Arm Trial for Evaluation of the Efficacy and Safety in the First Line Combination Therapy of Gemcitabine, Cisplatin and Nivolumab With Additional Pretreatment of AM80 for Urothelial Carcinoma Patients
【Treatment of Urothelial Carcinoma】 Treatment for urothelial carcinoma includes surgery, chemotherapy (anticancer drugs), and radiation therapy. Chemotherapy is generally used when metastasis has already occurred at diagnosis and surgery is not curative (metastatic urothelial carcinoma) or when the cancer recurs after local therapy such as surgery or radiation therapy (recurrent urothelial carcinoma).
Although there are several recommended treatments for urothelial carcinoma, the options are often limited by side effects and other factors, and these treatments may not be fully effective. Therefore, the development of safer and more effective treatments is desired.
【About the Drugs to be Used in this Clinical Trial】 In this clinical trial, the investigational drug MIKE-1 will be used in combination with nivolumab plus GC (cisplatin gemcitabine), one of the recommended chemotherapy regimens, and subsequently with nivolumab monotherapy for patients with unresectable metastatic or recurrent urothelial cancer. Nivolumab, cisplatin, and gemcitabine are injectable (intravenous infusion), while MIKE-1 is oral.
【Purpose of the Clinical Trial】 The purpose of this clinical trial is to evaluate the efficacy (how much the cancer shrinks or slows down) and safety of the investigational drug MIKE-1 in combination with nivolumab and gemcitabine and cisplatin therapy in patients with untreated unresectable or recurrent urothelial cancer.
Although there are several recommended treatments for urothelial carcinoma, the options are often limited by side effects and other factors, and these treatments may not be fully effective. Therefore, the development of safer and more effective treatments is desired.
【About the Drugs to be Used in this Clinical Trial】 In this clinical trial, the investigational drug MIKE-1 will be used in combination with nivolumab plus GC (cisplatin gemcitabine), one of the recommended chemotherapy regimens, and subsequently with nivolumab monotherapy for patients with unresectable metastatic or recurrent urothelial cancer. Nivolumab, cisplatin, and gemcitabine are injectable (intravenous infusion), while MIKE-1 is oral.
【Purpose of the Clinical Trial】 The purpose of this clinical trial is to evaluate the efficacy (how much the cancer shrinks or slows down) and safety of the investigational drug MIKE-1 in combination with nivolumab and gemcitabine and cisplatin therapy in patients with untreated unresectable or recurrent urothelial cancer.
Start Date01 May 2025 |
Sponsor / Collaborator  Nagoya University Nagoya University [+5] |
NCT06862596
A Multicenter, Randomized, Placebo-controlled, Double-blind Clinical Trial:
The Efficacy and Safety of Mexiletine Hydrochloride for Amelioration of Motor Dysfunction in Spinal and Bulbar Muscular Atrophy
The purpose of this clinical trial is to evaluate the efficacy and safety of mexiletine hydrochloride in patients with spinal and bulbar muscular atrophy.
The main questions it aims to answer are:
Does mexiletine hydrochloride improve the ALSFRS-R score in spinal and bulbar muscular atrophy patients?
Participants will:
Take mexiletine hydrochloride or a placebo every day for 3 months Visit the hospital once every 4 weeks for evaluations.
The main questions it aims to answer are:
Does mexiletine hydrochloride improve the ALSFRS-R score in spinal and bulbar muscular atrophy patients?
Participants will:
Take mexiletine hydrochloride or a placebo every day for 3 months Visit the hospital once every 4 weeks for evaluations.
Start Date28 Feb 2025 |
Sponsor / Collaborator |
JPRN-UMIN000055052
Influence of differences in flexibility on stretching effectiveness - Influence of differences in flexibility on stretching effectiveness
Start Date23 Jul 2024 |
Sponsor / Collaborator |
100 Clinical Results associated with Nagoya University
Login to view more data
0 Patents (Medical) associated with Nagoya University
Login to view more data
89,671
Literatures (Medical) associated with Nagoya University01 Feb 2026·NUTRITION
Extracellular water-to-total body water ratio and its relationship with activities of daily living in older inpatients in a convalescent setting
Article
Author: Akazawa, Naoki ; Okawa, Naomi ; Uchiyama, Yasushi ; Nagahiro, Shinji ; Hioka, Akemi
OBJECTIVE:
The aim of this study was to examine whether the extracellular water-to-total body water ratio (ECW/TBW) is associated with activities of daily living (ADLs) in older inpatients in a convalescent setting, and to determine whether ECW/TBW or skeletal muscle mass index (SMI) is more strongly correlated with ADLs.
DESIGN:
Retrospective study.
SETTING AND PARTICIPANTS:
A total of 196 older inpatients (86 males and 110 females) admitted to a convalescent rehabilitation ward were included.
METHODS:
The primary outcomes were ECW/TBW and ADLs at admission. ECW/TBW and SMI were measured using segmental multifrequency bioelectrical impedance analysis. ADLs were assessed with the Functional Independence Measure (FIM) motor score. Multiple linear regression analysis, adjusted for confounding factors, was performed to determine whether ECW/TBW was independently and significantly associated with FIM motor scores in male and female groups.
RESULTS:
The study cohort included 56 patients with stroke (28.6%), 45 with musculoskeletal disease (23.0%), and 95 with hospital-associated deconditioning (48.5%). The median (interquartile range; IQR) age was 81.0 (75.0-88.0) years in males and 87.0 (81.8-90.3) years in females. The median (IQR) ECW/TBW was 0.412 (0.404-0.421) in males and 0.419 (0.411-0.427) in females. The median (IQR) FIM motor score was 19.5 (14.0-39.0) in males and 23.0 (14.0-42.0) in females. In males, ECW/TBW was negatively associated with FIM motor scores, whereas SMI was positively associated. In females, ECW/TBW was negatively associated with FIM motor scores, whereas SMI was not significantly related.
CONCLUSIONS AND IMPLICATIONS:
ECW/TBW was negatively associated with ADLs in older inpatients in a convalescent setting in both sexes. Although SMI was positively associated with ADLs in males, no such relationship was observed in females. These findings suggest that monitoring ECW/TBW may be a useful approach for evaluating ADLs in older inpatients.
01 Feb 2026·JOURNAL OF CRITICAL CARE
Recovery of motor functions and cognitive functions in patients with intensive care unit–acquired weakness
Article
Author: Hoshiyama, Minoru ; Suzuki, Shogo ; Umikawa, Mako ; Aoyama, Yusuke ; Okazaki, Masaki
PURPOSE:
This study aimed to identify factors influencing recovery of activities of daily living (ADLs) in patients with ICU-acquired weakness (ICU-AW) after ICU discharge.
METHODS:
A retrospective cohort study was conducted with 65 ICU-AW patients. Data were collected from ICU discharge to four weeks post-discharge. Variables analyzed included age, sex, APACHE-II score, mechanical ventilation duration, and presence of delirium. The primary outcome was ADL recovery. Multiple regression analysis was performed using data at ICU discharge, and a linear mixed model assessed changes in motor Functional Independence Measure (FIM) scores.
RESULTS:
Median age was 61 years (IQR: 50-70), with females comprising 44.6 %. Median APACHE-II score was 25 (IQR: 20-29), and 69.2 % were surgical ICU patients. Median mechanical ventilation duration was 19 days (IQR: 10-34), and delirium lasted a median of three days (IQR: 1-7). Multivariable analysis showed cognitive FIM score at ICU discharge (β = 19.12, p = 0.001) and delirium duration (β = -9.83, p < 0.001) were independently associated with ADL recovery. The linear mixed model revealed a significant difference in motor FIM gain between patients with preserved cognition and those with cognitive dysfunction (p = 0.001).
CONCLUSIONS:
Cognitive function at ICU discharge and delirium duration significantly predicted ADL recovery. These factors should be considered in post-ICU rehabilitation planning.
01 Jan 2026·METABOLISM-CLINICAL AND EXPERIMENTAL
Glucoprivation-induced nutrient preference relies on distinct NPY neurons that project to the paraventricular nucleus of the hypothalamus
Article
Author: Minokoshi, Yasuhiko ; Kobayashi, Kenta ; Nakajima, Ken-Ichiro ; Kondoh, Kunio ; Rattanajearakul, Nawarat ; Fu, Ou ; Okamoto, Shiki
BACKGROUND:
Neural pathways related to total calorie intake have been extensively studied. However, it remains unclear how these mechanisms control food selection.
METHODS:
Male mice were subjected to glucoprivation through the intraperitoneal (i.p.) administration of 2-deoxy-d-glucose (2DG) and were examined for food selection between a high-carbohydrate diet (HCD) and a high-fat diet (HFD) in a diet choice paradigm. This involved the chemogenetic or optogenetic modulation of the neural activity of AMP-activated protein kinase (AMPK)-regulated corticotropin-releasing hormone (CRH) neurons, melanocortin-4 receptor (MC4R) neurons in the paraventricular nucleus of the hypothalamus (PVH), and neuropeptide Y (NPY) neurons projecting to the PVH.
RESULTS:
Glucoprivation induced by 2DG administration in mice influenced two distinct neural pathways in the PVH that separately promote the intake of an HCD or an HFD. Injection of 2DG activated PVH-projecting NPY neurons in the nucleus of the solitary tract (NTS) and ventrolateral medulla (VLM), resulting in a rapid increase in HCD intake through stimulation of PVH AMPK-regulated CRH neurons and recovery from glucoprivation. In contrast, PVH-projecting NPY neurons in the NTS, VLM, and arcuate nucleus of the hypothalamus (ARC) promoted HFD intake by inhibiting MC4R neurons in the PVH, reflecting the strong innate preference for an HFD in mice. The ARC NPY neurons specifically promoted HFD selection.
CONCLUSION:
Our findings reveal a previously unrecognized mechanism for food selection between HCD and HFD during glucoprivation.
79
News (Medical) associated with Nagoya University09 Sep 2025
GEXVal Inc. June 9, 2025
GEXVal Inc. (President and CEO: Juran Kato, PhD; Location: Fujisawa, Kanagawa, Japan, hereinafter “GEXVal”) is pleased to announce that Chief Technology Officer Dr. Yusuke Nakayama presented at a seminar hosted by Nagoya University Graduate School of Pharmaceutical Sciences, delivering a lecture titled "Drug Discovery Career Paths through Bioinformatics: From Pharmaceutical Companies to Ventures, and AI Applications."
[Seminar Overview] Dr. Yusuke Nakayama brings over 20 years of extensive practical experience spanning from major pharmaceutical companies to drug discovery ventures, with expertise across diverse fields including target identification, drug repurposing, system development, and open innovation. In this seminar, he shared insights on organizational characteristics and key considerations for career development, while also introducing the latest trends in AI applications in drug discovery and platform development cases utilizing large language models (LLMs).
**Presentation Details**
Title
Drug Discovery Career Paths through Bioinformatics: From Pharmaceutical Companies to Ventures, and AI Applications
Date
September 2, 2025 (Tuesday)
Venue
Nagoya University Graduate School of Pharmaceutical Sciences
[About Nagoya University] Nagoya University, established in 1939, is a prestigious national research university in Japan with seven Nobel Prize winners associated with it. The Graduate School of Informatics integrates natural sciences, social sciences, and humanities to develop cutting-edge information science that contributes to an advanced information-oriented society. Ranked among the top 200 universities globally*, the university is renowned for its innovative research and international programs.
For more information, visit: https://en.nagoya-u.ac.jp
*Reference: QS Quacquarelli Symonds Limited, TopUniversities URL (as of September 4, 2025): https://www.topuniversities.com/universities/nagoya-university
[About GEXVal] GEXVal strives to create and develop innovative pharmaceuticals for unmet medical needs, ensuring Treatment Reaches the Unreached with focus on rare diseases and underserved medical conditions. By leveraging our proprietary AI-powered pharmacoinformatics technology, we illuminate paths to breakthrough therapies, identifying hidden potential in drug candidates to deliver life-changing medicines that bring new hope to patients and their families.
For further information: Head of Corporate Office Atsushi Sugizaki info@gexval.com
15 Jul 2025
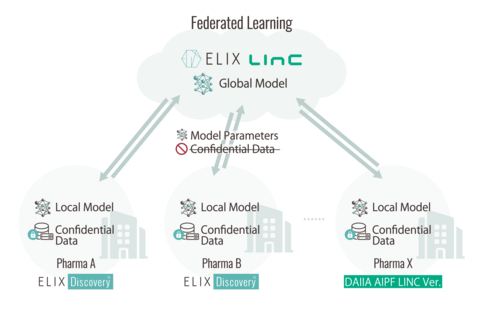
TOKYO--(BUSINESS WIRE)--Elix, Inc. (CEO: Shinya Yuki / Headquarters: Tokyo, hereinafter “Elix”), an AI drug discovery company with the mission of "Rethinking Drug Discovery” and the Life Intelligence Consortium (Representative Director: Yasushi Okuno / Headquarters: Osaka, hereinafter “LINC”) are pleased to announce that for the first time in the world we have commercialized an AI drug discovery platform that incorporates multiple AI models trained using federated learning on data provided by 16 pharmaceutical companies.
The key to AI drug discovery lies in high-quality and sufficiently large datasets. Diverse and abundant data are indispensable for building superior AI models; however, pharmaceutical companies are generally limited to utilizing their own proprietary data and public datasets, resulting in significant data shortages that have posed major challenges to progress.
Federated learning technology provides a solution to this challenge. Elix, in partnership with the Department of Biomedical Data Intelligence, Graduate School of Medicine, Kyoto University, developed the federated learning library kMoL*¹, enabling multiple companies to collaboratively develop a suite of AI models without disclosing their confidential data externally. 16 pharmaceutical companies participated in building these federated learning-based models, which are now implemented on Elix Discovery™, Elix’s proprietary AI drug discovery platform.
By introducing Elix Discovery™, users can immediately leverage these newly developed models, and several pharmaceutical companies have already adopted the platform. The number of adoptions is expected to expand further, and Elix Discovery™ is on track to become the de-facto standard for AI drug discovery in Japan. Moreover, this initiative marks the world’s first commercialization of an AI drug discovery platform in partnership with numerous pharmaceutical companies utilizing federated learning.
The development of federated learning-based AI models*² was advanced through “Development of a Next-generation Drug Discovery AI through Industry-academia Collaboration” (DAIIA), an industry-academia collaborative program under the Project Promoting Support for Drug Discovery led by the Japan Agency for Medical Research and Development (AMED). Launched in FY2020 with the aim of establishing a drug discovery support infrastructure leveraging AI, the project involved 17 pharmaceutical companies, research institutes such as RIKEN, Kyoto University, Nagoya University, along with about 10 IT companies with AI expertise. The project concluded at the end of March 2025.
To ensure the continued operation and advancement of the innovative models and mechanisms cultivated in DAIIA, Elix -- which already operates its own AI drug discovery platform -- and LINC -- a consortium supporting industry-academia collaboration in AI for life sciences with participation from many DAIIA member companies, have joined forces to commence the world’s first commercialization of an AI drug discovery platform serving models pre-trained with federated data, subsequently starting from April 2025. Through this initiative, we expect to see further adoptions of these technologies in real-world drug discovery settings.
Initially, the primary users will be the pharmaceutical companies that participated in DAIIA. However, as more companies join, the pool of available data will expand, further improving the accuracy and usability of the AI models for all users. We also plan to actively open the platform to companies that were not part of DAIIA. If you are interested in this initiative, please reach out to us via the contact information below.
*¹ For more information on kMoL: https://www.elix-inc.com/news/newsrelease/1470/
*² R&D project title: “Development of an integrated drug discovery AI platform combining multi-target prediction and structure generation models using state-of-the-art AI technologies”
https://www.amed.go.jp/program/list/11/02/001_02-04.html
Comment from Yasushi Okuno, Ph.D., Representative Director, LINC; Professor, Department of Biomedical Data Intelligence, Graduate School of Medicine, Kyoto University; Division Director, HPC- and AI-driven Drug Development Platform Division, Center for Computational Science, RIKEN ; and Co-Investigator of the AMED DAIIA Project
On the occasion of commercializing the AMED project, I would like to express my heartfelt gratitude to AMED and to all the pharmaceutical companies that have cooperated with us.
This commercialization has two points of significance. First, while many government-funded projects fail to reach practical implementation after their funding period ends, this initiative will be utilized by pharmaceutical companies, thereby contributing directly to real-world drug discovery. Second, multiple pharmaceutical companies will continue to share data across the industry through federated learning, aiming to develop highly accurate AI. In an industry where the pursuit of individual corporate profit often takes precedence, the effort by each of these companies to share data for the benefit of patients and to build and utilize high-performance drug-discovery AI is profoundly meaningful and a source of great pride.
I sincerely hope that this project will become a cornerstone for enhancing Japan’s drug-discovery capabilities and, ultimately, contribute to the health of patients around the world.
Comment from Teruki Honma, Ph.D., Team Director, Molecular Design Control Research Team, Center for Biosystems Dynamics Research, RIKEN; and R&D Principal Investigator of the AMED DAIIA Project
I am delighted that the AI models for on/off-target prediction, ADMET prediction, and molecular generation produced by the AMED DAIIA project will now be commercialized by LINC and Elix.
DAIIA’s predictive AIs were built using chemical structure data provided by pharmaceutical companies, and training on structural data covering more than 1 million compounds and over 10 million data points, is unprecedented on a global scale. This training was made possible by a dedicated system capable of stable federated learning, allowing collaborative model development while preserving confidentiality.
Regarding the generative AI, we plan to extend ChemTS and incorporate advanced functions such as DyRAMO, which enables efficient multi-objective optimization. This will make it possible to create novel compounds and evaluate their activity profiles with higher accuracy and speed than ever before.
Continuous updates are indispensable when leveraging technologies such as federated learning and generative AI. Through this commercialization, I expect the achievements of DAIIA to keep evolving and, delivered as long-term software for pharmaceutical companies, to greatly contribute to the acceleration and innovation of drug discovery research.
Comment from Shinya Yuki, Ph.D., Co-Founder and CEO, Elix
Data scarcity remains one of the biggest challenges in AI drug discovery. By jointly developing federated learning technology, kMoL, with the Department of Biomedical Data Intelligence, Graduate School of Medicine, Kyoto University, we have created a system that enables learning from the data held by 16 pharmaceutical companies while preserving the confidentiality of data such as compound structures.
Commercializing the predictive models we have built and deploying them on an AI drug discovery platform is a world-first initiative. This accomplishment was only possible through the collaboration of pharmaceutical companies, academia, AMED, LINC, and AI/IT enterprises involved in the project, and represents an important milestone that advances the use of AI in the pharmaceutical industry to a new stage. I believe that this will make Elix Discovery™ the de-facto standard of AI drug discovery platforms.
This federated learning based initiative is just the starting point for further progress. By encouraging even greater participation and data contributions from pharmaceutical companies, we aim to further expand and strengthen this initiative, enhancing our contribution to the pharmaceutical industry as a whole and ultimately to patients.
About LINC
The Life Intelligence Consortium aims to advance and implement applications of AI, big data, IoT, and related technologies in the life-science field, promoting the field’s development, human-resource cultivation, digital transformation, and economic growth. For details, please visit https://linc-ai.jp.
About Elix
Elix is an AI drug discovery company with the mission of “Rethinking drug discovery”. Through our flagship software platform, Elix Discovery™, and collaborative consulting engagements, we work with leading pharmaceutical companies and biotech startups to reduce the enormous costs and time associated with drug discovery, while improving the rate of successful outcomes. We achieve these goals via our company’s unique blend of AI engineering and medicinal chemistry expertise that allows us to stay at the forefront of development in both fields, and offer the solutions of tomorrow to the problems of today.
https://www.elix-inc.com/
21 Mar 2025
FRIDAY, March 21, 2025 -- For individuals with
type 1 diabetes
, low-glucose alerts improve the time below range for drivers and reduce the incidence of low glucose while driving, according to a study published in the April issue of
Diabetes Research and Clinical Practice
.
Ryutaro Maeda, from Nagoya University Graduate School of Medicine in Japan, and colleagues examined the effectiveness of continuous glucose monitoring (CGM) with low-glucose alerts for preventing hypoglycemia in insulin-treated drivers with diabetes in a single-center, open-label, randomized crossover study. Thirty insulin-treated participants with diabetes who drove cars at least three times per week were enrolled and underwent two four-week periods: an alert period using CGM with active low-glucose alerts and a no-alert period using blinded CGM without low-glucose alerts.
Twenty-seven participants completed the analysis. The researchers found that among all participants, the time below range (<3.9 mmol/L) did not differ between the alert and no-alert periods, but among participants with type 1 diabetes, the time below range decreased significantly during the alert period (treatment difference, −4.4). Compared with the no-alert period, the incidence of low-glucose when driving was significantly lower during the alert period (19 versus 33 percent).
"CGM with low-glucose alerts has shown potential to reduce the risk of hypoglycemia for insulin-treated drivers," the authors write. "These systems are expected to make driving safer for people with diabetes."
Several authors disclosed ties to Abbott Diabetes Care, which funded the study.
Abstract/Full Text
Whatever your topic of interest,
subscribe to our newsletters
to get the best of Drugs.com in your inbox.
Clinical ResultAHA
100 Deals associated with Nagoya University
Login to view more data
100 Translational Medicine associated with Nagoya University
Login to view more data
Corporation Tree
Boost your research with our corporation tree data.
login
or

Pipeline
Pipeline Snapshot as of 03 Mar 2026
The statistics for drugs in the Pipeline is the current organization and its subsidiaries are counted as organizations,Early Phase 1 is incorporated into Phase 1, Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3
Discovery
3
18
Preclinical
Phase 1
2
1
Phase 3
Other
16
Login to view more data
Current Projects
| Drug(Targets) | Indications | Global Highest Phase |
|---|---|---|
Adipose derived stem cells (Assistance Publique Hôpitaux de Marseille) | Scleroderma, Systemic More | Phase 3 |
Nek2 siRNA therapy(Nagoya University) ( NEK2 ) | Bile Duct Neoplasms More | Phase 1 |
PiggyBac transposon-mediated CD-19 CAR T-cells ( CD19 ) | Acute Lymphoblastic Leukemia More | Phase 1 |
GCT-102 | Spinal Cord Injuries More | Preclinical |
CD19/CD37 CAR-T (Nagoya University) ( CD19 x CD37 ) | Neoplasms More | Preclinical |
Login to view more data
Deal
Boost your decision using our deal data.
login
or

Translational Medicine
Boost your research with our translational medicine data.
login
or

Profit
Explore the financial positions of over 360K organizations with Synapse.
login
or

Grant & Funding(NIH)
Access more than 2 million grant and funding information to elevate your research journey.
login
or

Investment
Gain insights on the latest company investments from start-ups to established corporations.
login
or

Financing
Unearth financing trends to validate and advance investment opportunities.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
