Request Demo
Last update 06 Dec 2025

The Chinese University of Hong Kong
Last update 06 Dec 2025
Overview
Tags
Neoplasms
Other Diseases
Endocrinology and Metabolic Disease
Small molecule drug
Fusion protein
mRNA
Disease domain score
A glimpse into the focused therapeutic areas
No Data
Technology Platform
Most used technologies in drug development
No Data
Targets
Most frequently developed targets
No Data
| Top 5 Drug Type | Count |
|---|---|
| Small molecule drug | 15 |
| Herbal medicine | 2 |
| mRNA | 1 |
| miRNA | 1 |
| Macrophage therapy | 1 |
| Top 5 Target | Count |
|---|---|
| HPV E6 | 1 |
| SARS-CoV-2 M protein | 1 |
| CNBP x MTDH | 1 |
| HDAC8(Histone deacetylase 8) | 1 |
| FOXP4(forkhead box P4) | 1 |
Related
31
Drugs associated with The Chinese University of Hong KongTarget |
Mechanism glucokinase activators |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc. China |
First Approval Date30 Sep 2022 |
Mechanism CACNA1G blockers [+2] |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication- |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc.- |
First Approval Date- |
Target |
Mechanism 30S subunit inhibitors |
Active Org. |
Originator Org.- |
Inactive Indication |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc.- |
First Approval Date01 Jan 1959 |
1,853
Clinical Trials associated with The Chinese University of Hong KongNCT06676371
Randomized Control Trial of Total Arch Replacement With Frozen Elephant Trunk Versus Conventional Hemiarch Replacement in Patients With Acute Type A Aortic Dissection (TARFET-TAAD)
Acute Type A aortic dissection (ATAAD) and its complications are life-threatening conditions that cause more than 1400 hospital admissions and 300 deaths every year in Hong Kong and cause 172,927 deaths globally in 2019. There is an increasing trend in recent years.
Given the hyperacute presentation and complexity of the clinical manifestation with various mal-perfusion syndromes, the mortality of ATAAD remained high in all international reported registries and published series.
Conventional Hemiarch (HAR) replacement has been the gold standard procedure for ATAAD in most of cardiac surgical centers around the world because of the relative simplicity of the procedure. However, around 50-70% of patients were reported to develop distal anastomosis new entry after hemiarch procedure and for those with entry tear over aortic arch and distal mal-perfusion, hemiarch alone might not be able to solve the downstream problem. On the other hand, total arch replacement and frozen elephant trunk procedure (TARFET) is more complex, technically demanding procedure that could potentially cover/resect the arch entry tear and exclude tear over proximal descending thoracic aorta and, hence, solve the distal mal-perfusion syndrome. To date, there is no randomized control trial to answer whether HAR or TARFET procedure is superior in patients with ATAAD and entry tear is over the aortic arch or proximal descending thoracic aorta.
We plan to conduct a multi-center trial to recruit consecutive patients with ATAAD with entry tear beyond ascending aorta and randomized them, after informed consent, into either the conventional hemiarch replacement group (HAR) or total arch replacement and frozen elephant trunk (TARFET). The participating centers will collect pre- operative, intra-operative and post-operative clinical parameters for two groups of patients via REDCap system. Written informed consent, specifically allowing the use of clinical records for this randomized study, will be obtained from every patient prior to data collection. The primary outcome is the 30-day mortality of the ATAAD patients with surgically treated by HAR versus TARFET approach. The secondary outcomes are the major adverse cardiovascular and cerebral events, post-op renal replacement therapy, re-exploration for bleeding and re-intervention within 30-days.
This study will be the world's first multi-center randomized control trial in ATAAD to compare the 30-day mortality of patients treated with HAR and TARFET. It could be a guideline-changing study for the treatment of ATAAD and its impact on the surgical approach to patients suffering from ATAAD.
Given the hyperacute presentation and complexity of the clinical manifestation with various mal-perfusion syndromes, the mortality of ATAAD remained high in all international reported registries and published series.
Conventional Hemiarch (HAR) replacement has been the gold standard procedure for ATAAD in most of cardiac surgical centers around the world because of the relative simplicity of the procedure. However, around 50-70% of patients were reported to develop distal anastomosis new entry after hemiarch procedure and for those with entry tear over aortic arch and distal mal-perfusion, hemiarch alone might not be able to solve the downstream problem. On the other hand, total arch replacement and frozen elephant trunk procedure (TARFET) is more complex, technically demanding procedure that could potentially cover/resect the arch entry tear and exclude tear over proximal descending thoracic aorta and, hence, solve the distal mal-perfusion syndrome. To date, there is no randomized control trial to answer whether HAR or TARFET procedure is superior in patients with ATAAD and entry tear is over the aortic arch or proximal descending thoracic aorta.
We plan to conduct a multi-center trial to recruit consecutive patients with ATAAD with entry tear beyond ascending aorta and randomized them, after informed consent, into either the conventional hemiarch replacement group (HAR) or total arch replacement and frozen elephant trunk (TARFET). The participating centers will collect pre- operative, intra-operative and post-operative clinical parameters for two groups of patients via REDCap system. Written informed consent, specifically allowing the use of clinical records for this randomized study, will be obtained from every patient prior to data collection. The primary outcome is the 30-day mortality of the ATAAD patients with surgically treated by HAR versus TARFET approach. The secondary outcomes are the major adverse cardiovascular and cerebral events, post-op renal replacement therapy, re-exploration for bleeding and re-intervention within 30-days.
This study will be the world's first multi-center randomized control trial in ATAAD to compare the 30-day mortality of patients treated with HAR and TARFET. It could be a guideline-changing study for the treatment of ATAAD and its impact on the surgical approach to patients suffering from ATAAD.
Start Date01 Oct 2026 |
Sponsor / Collaborator |
NCT07230457
Prospective Evaluation of Plasma Mycobacterium Tuberculosis Cell-free DNA Sequencing as a Non-Invasive Alternative to Bronchoscopy for Diagnosing Pulmonary Tuberculosis
Tuberculosis (TB) remains a major global health challenge, affecting over 10 million people annually. Hong Kong carries an intermediate TB burden, with
3,200 new cases reported yearly. Pulmonary TB (PTB), the most common form, presents diagnostic difficulties. Traditional methods like sputum smear and culture often fail in patients unable to produce adequate samples, necessitating bronchoscopy to collect bronchoalveolar lavage (BAL) for mycobacterial testing.
These limitations pose risks for patients and strain healthcare systems. Bronchoscopy is invasive, resource-intensive, and may delay treatment-especially for elderly patients with comorbidities. Blood-based inflammatory markers lack diagnostic specificity. A rapid, non-invasive alternative is urgently needed.
The investigators developed a plasma-based assay that detects Mycobacterium tuberculosis cell-free DNA (MTB cfDNA) in blood. This liquid biopsy leverages metagenomic sequencing and computational analysis to identify TB-specific genetic material while minimizing contamination. Preliminary data show excellent diagnostic performance, with area under the receiver operating characteristic curve values >0.94 for TB pleurisy.
The investigators propose a prospective clinical validation study comparing plasma MTB cfDNA testing to bronchoscopy with BAL culture and molecular testing. The primary aim is to demonstrate non-inferiority of plasma cfDNA within a 10% sensitivity margin. Secondary aims include assessing how clinical and radiological features affect test performance and evaluating the assay's ability to detect drug resistance mutations for personalized therapy.
Validation could transform TB diagnosis by offering a rapid, safe, and accurate blood test. Patients could avoid invasive procedures, receive faster diagnoses, and begin treatment sooner. Detecting resistance mutations directly from plasma would enable timely, targeted therapy-critical for addressing multidrug-resistant TB. This represents a paradigm shift toward precision medicine in TB care.
Tailored to Hong Kong's epidemiological context, this study addresses a key diagnostic gap. The approach has global relevance, with potential to improve clinical outcomes, reduce costs, and accelerate progress toward WHO's TB elimination goals.
3,200 new cases reported yearly. Pulmonary TB (PTB), the most common form, presents diagnostic difficulties. Traditional methods like sputum smear and culture often fail in patients unable to produce adequate samples, necessitating bronchoscopy to collect bronchoalveolar lavage (BAL) for mycobacterial testing.
These limitations pose risks for patients and strain healthcare systems. Bronchoscopy is invasive, resource-intensive, and may delay treatment-especially for elderly patients with comorbidities. Blood-based inflammatory markers lack diagnostic specificity. A rapid, non-invasive alternative is urgently needed.
The investigators developed a plasma-based assay that detects Mycobacterium tuberculosis cell-free DNA (MTB cfDNA) in blood. This liquid biopsy leverages metagenomic sequencing and computational analysis to identify TB-specific genetic material while minimizing contamination. Preliminary data show excellent diagnostic performance, with area under the receiver operating characteristic curve values >0.94 for TB pleurisy.
The investigators propose a prospective clinical validation study comparing plasma MTB cfDNA testing to bronchoscopy with BAL culture and molecular testing. The primary aim is to demonstrate non-inferiority of plasma cfDNA within a 10% sensitivity margin. Secondary aims include assessing how clinical and radiological features affect test performance and evaluating the assay's ability to detect drug resistance mutations for personalized therapy.
Validation could transform TB diagnosis by offering a rapid, safe, and accurate blood test. Patients could avoid invasive procedures, receive faster diagnoses, and begin treatment sooner. Detecting resistance mutations directly from plasma would enable timely, targeted therapy-critical for addressing multidrug-resistant TB. This represents a paradigm shift toward precision medicine in TB care.
Tailored to Hong Kong's epidemiological context, this study addresses a key diagnostic gap. The approach has global relevance, with potential to improve clinical outcomes, reduce costs, and accelerate progress toward WHO's TB elimination goals.
Start Date01 Oct 2026 |
Sponsor / Collaborator |
NCT07256457
Multimodal Machine Learning Integration of Pulmonary Function Parameters and 3D-CT Imaging for Postoperative Outcome Prediction in Early-Stage NSCLC
Improvements in low-dose CT screening have led to an increase in early-stage NSCLC diagnoses, with surgical resection-usually lobectomy or segmentectomy-remaining the primary curative option. In Hong Kong, however, patients frequently present with comorbidities such as chronic respiratory disease or cardiovascular issues, making the preservation of healthy lung tissue crucial for their long-term quality of life. Traditional surgical resection, such as lobectomy or segmentectomy, has certain limitations in terms of functional preservation. In contrast, robotic/navigational bronchoscopic ablation has emerged in recent years as a novel minimally invasive endoscopic treatment strategy. This approach has been implemented in select centers and demonstrates potential advantages, including faster postoperative recovery, reduced trauma, and improved preservation of pulmonary function. By leveraging advanced navigation systems, bronchoscopic ablation enables precise localization and ablation of pulmonary nodules, avoiding the extensive resection of healthy lung tissue required in traditional surgery. These benefits hold promise for enhancing patients' long-term quality of life and survival rates.
Moreover, conventional pulmonary function tests like FEV₁ and diffusing capacity of the lung for carbon monoxide provide only a global assessment of respiratory capacity, which may not fully capture the regional changes in pulmonary function that occur following segmentectomy or lobectomy. Likewise, basic CT volumetry overlooks finer anatomical details such as segmental airway distribution, microvascular networks, and local alveolar compliance. Furthermore, there is currently a paucity of direct comparative studies between robotic/navigational bronchoscopic ablation and traditional surgical resection regarding postoperative pulmonary function and long-term outcomes. Supported by Research Grants Council, our work since 2019 has validated the feasibility and safety of this technique, leading to widespread recognition and numerous publications. However, most existing research is retrospective or derived from single-center data, with a primary focus on short-term safety and technical feasibility.
To address these limitations, an integrative approach leveraging 3D-CT imaging and ML is proposed. Machine learning is a technology that uses algorithms to automatically learn from data and make predictions or decisions. Deep learning, a subfield of ML, utilizes multi-layer neural networks to effectively extract features and recognize patterns in complex, high-dimensional data. ML, and particularly DL, has demonstrated remarkable potential in various medical imaging applications, including lesion detection, tissue segmentation, and outcome prediction. By automatically learning complex patterns in high-dimensional data, ML and DL models can interpret subtle radiologic characteristics that may be missed by conventional analyses. In the context of 3D-CT imaging for NSCLC, DL architectures-such as convolutional neural networks-can extract detailed features from volumetric scans, enabling robust quantification of tumor size, shape, and location as well as refined assessment of lung parenchyma. When integrated with pulmonary function parameters and clinical data, these algorithms provide a powerful means to generate predictive models, identify at-risk patients earlier, and guide individualized treatment planning. Moreover, ML-driven approaches can adapt to evolving datasets over time, continuously refining and improving their performance. This scalability and adaptability are especially valuable in prospective studies, where large, multimodal datasets are collected to evaluate the long-term impact of different treatment strategies. Consequently, incorporating ML and DL in this research not only enhances the precision of outcome prediction but also contributes to a standardized framework for dynamic, personalized assessment of pulmonary function, guiding more informed clinical decision-making.
The primary aim of this study is to determine whether segmentectomy truly offers better functional preservation than lobectomy, whether robotic/navigation-guided bronchoscopic ablation indeed achieves superior pulmonary function preservation compared to traditional surgical resection, and under which specific patient conditions each approach may yield the greatest benefit. By undertaking a prospective, well-designed investigation, the research will fill a critical gap in evidence regarding long-term functional outcomes, providing clearer criteria for selecting the most appropriate resection type. Moreover, the introduction of a standardized, integrative assessment tool has the potential to optimize surgical decision-making and postoperative care, ultimately improving survival and quality of life for early-stage NSCLC patients in Hong Kong and potentially informing best practices in other healthcare contexts.
Moreover, conventional pulmonary function tests like FEV₁ and diffusing capacity of the lung for carbon monoxide provide only a global assessment of respiratory capacity, which may not fully capture the regional changes in pulmonary function that occur following segmentectomy or lobectomy. Likewise, basic CT volumetry overlooks finer anatomical details such as segmental airway distribution, microvascular networks, and local alveolar compliance. Furthermore, there is currently a paucity of direct comparative studies between robotic/navigational bronchoscopic ablation and traditional surgical resection regarding postoperative pulmonary function and long-term outcomes. Supported by Research Grants Council, our work since 2019 has validated the feasibility and safety of this technique, leading to widespread recognition and numerous publications. However, most existing research is retrospective or derived from single-center data, with a primary focus on short-term safety and technical feasibility.
To address these limitations, an integrative approach leveraging 3D-CT imaging and ML is proposed. Machine learning is a technology that uses algorithms to automatically learn from data and make predictions or decisions. Deep learning, a subfield of ML, utilizes multi-layer neural networks to effectively extract features and recognize patterns in complex, high-dimensional data. ML, and particularly DL, has demonstrated remarkable potential in various medical imaging applications, including lesion detection, tissue segmentation, and outcome prediction. By automatically learning complex patterns in high-dimensional data, ML and DL models can interpret subtle radiologic characteristics that may be missed by conventional analyses. In the context of 3D-CT imaging for NSCLC, DL architectures-such as convolutional neural networks-can extract detailed features from volumetric scans, enabling robust quantification of tumor size, shape, and location as well as refined assessment of lung parenchyma. When integrated with pulmonary function parameters and clinical data, these algorithms provide a powerful means to generate predictive models, identify at-risk patients earlier, and guide individualized treatment planning. Moreover, ML-driven approaches can adapt to evolving datasets over time, continuously refining and improving their performance. This scalability and adaptability are especially valuable in prospective studies, where large, multimodal datasets are collected to evaluate the long-term impact of different treatment strategies. Consequently, incorporating ML and DL in this research not only enhances the precision of outcome prediction but also contributes to a standardized framework for dynamic, personalized assessment of pulmonary function, guiding more informed clinical decision-making.
The primary aim of this study is to determine whether segmentectomy truly offers better functional preservation than lobectomy, whether robotic/navigation-guided bronchoscopic ablation indeed achieves superior pulmonary function preservation compared to traditional surgical resection, and under which specific patient conditions each approach may yield the greatest benefit. By undertaking a prospective, well-designed investigation, the research will fill a critical gap in evidence regarding long-term functional outcomes, providing clearer criteria for selecting the most appropriate resection type. Moreover, the introduction of a standardized, integrative assessment tool has the potential to optimize surgical decision-making and postoperative care, ultimately improving survival and quality of life for early-stage NSCLC patients in Hong Kong and potentially informing best practices in other healthcare contexts.
Start Date01 Jul 2026 |
Sponsor / Collaborator |
100 Clinical Results associated with The Chinese University of Hong Kong
Login to view more data
0 Patents (Medical) associated with The Chinese University of Hong Kong
Login to view more data
53,893
Literatures (Medical) associated with The Chinese University of Hong Kong01 Dec 2026·Nano-Micro Letters
Boron-Insertion-Induced Lattice Engineering of Rh Nanocrystals Toward Enhanced Electrocatalytic Conversion of Nitric Oxide to Ammonia
Article
Author: Han, Peng ; Ling, Chongyi ; Xu, Lei ; Wang, Jinlan ; Ma, Chen ; Gu, Zhengxiang ; Xu, Xiangou ; Gu, Ping ; Chen, Weiwei ; Zheng, Long ; He, Qiyuan ; Su, Dong ; Chen, Ye ; Zeng, Zhiyuan ; Wang, Gang ; Wang, Wenbin
Abstract:
Electrocatalytic nitric oxide (NO) reduction reaction (NORR) is a promising and sustainable process that can simultaneously realize green ammonia (NH3) synthesis and hazardous NO removal. However, current NORR performances are far from practical needs due to the lack of efficient electrocatalysts. Engineering the lattice of metal-based nanomaterials via phase control has emerged as an effective strategy to modulate their intrinsic electrocatalytic properties. Herein, we realize boron (B)-insertion-induced phase regulation of rhodium (Rh) nanocrystals to obtain amorphous Rh4B nanoparticles (NPs) and hexagonal close-packed (hcp) RhB NPs through a facile wet-chemical method. A high Faradaic efficiency (92.1 ± 1.2%) and NH3 yield rate (629.5 ± 11.0 µmol h−1 cm−2) are achieved over hcp RhB NPs, far superior to those of most reported NORR nanocatalysts. In situ spectro-electrochemical analysis and density functional theory simulations reveal that the excellent electrocatalytic performances of hcp RhB NPs are attributed to the upshift of d-band center, enhanced NO adsorption/activation profile, and greatly reduced energy barrier of the rate-determining step. A demonstrative Zn–NO battery is assembled using hcp RhB NPs as the cathode and delivers a peak power density of 4.33 mW cm−2, realizing simultaneous NO removal, NH3 synthesis, and electricity output.
01 Apr 2026·BIOMATERIALS
Tumor microenvironment-responsive NanoShield for precision photodynamic and photothermal synergistic cancer therapy with mitigated skin phototoxicity
Article
Author: Wang, Wen-Jin ; Tang, Ben Zhong ; Zhang, Rongyuan ; Zhu, Yilin ; Tan, Haozhe ; Yang, Han ; Zhao, Zheng ; Zhang, Liping ; Xiong, Yu
Photodynamic therapy (PDT) is a highly promising non-invasive cancer treatment but is often hampered by severe skin phototoxicity arising from the nonspecific distribution of photosensitizers (PSs). Stimuli-responsive aggregation-induced emission (AIE) PSs show great promise for enhancing selectivity and efficacy. However, challenges such as complex synthesis and inadequate in vivo phototoxicity evaluations still need to be addressed. Furthermore, single-modality PDT frequently yields suboptimal therapeutic outcomes. To address these limitations, we present a tumor microenvironment (TME)-responsive AIE NanoShield (P-AD@PD), a precision theranostic nanoplatform engineered for synergistic phototherapy with mitigated skin phototoxicity. The NanoShield comprises a poly(N-isopropylacrylamide-co-acrylic acid) (PNA) nanogel system encapsulating an AIE PS (AD), shielded by a polydopamine (PDA) coating to enhance photoprotection. During systemic circulation, the PDA layer quenches AD photoactivity, minimizing skin phototoxicity. Upon tumor accumulation, it triggers hyperthermia for photothermal therapy (PTT) and gradually degrades within the TME to reactivate AD for precise PDT. This sequential PTT-PDT regimen amplifies therapeutic efficacy through dual-mode imaging while mitigating off-target toxicity. The PDA-based shielding strategy offers broad applicability across PSs, providing a universal approach to enhance PDT efficacy and safety.
01 Apr 2026·BIOMATERIALS
Inflammation-targeted nanoplatform: NIR-II imaging-guided encephalitis suppressing by dual antioxidant-ferroptosis action
Article
Author: Wang, Qianruo ; Tang, Ben Zhong ; Li, Yuanyuan ; Wang, Di ; Liu, Penghui ; Yin, Xin
Encephalitis, a life-threatening neurological disorder with high mortality and debilitating long-term sequelae, remains a formidable clinical challenge due to limited therapeutic strategies targeting its underlying pathological mechanisms. Current interventions, constrained by blood-brain barrier (BBB) impermeability and a focus on symptomatic relief, fail to mitigate ferroptosis and reactive oxygen species (ROS)-mediated neurotoxicity-central drivers of disease progression. Here, we present CeO2/3TT@NP-RVG, a multifunctional nanomaterial engineered for integrated diagnosis and treatment of encephalitis. The nanoplatform combines ROS-scavenging cerium oxide (CeO2) with photothermal NIR-II-emissive (3TT) nanoparticles, enabling real-time fluorescence imaging of encephalitis with deep-tissue resolution. Functionalization with rabies virus glycoprotein-derived RVG peptide ensures BBB penetration and neuron-targeted delivery. In LPS-induced encephalitis models, CeO2/3TT@NP-RVG demonstrated dual therapeutic efficacy: alleviating oxidative stress by neutralizing ROS, suppressing pro-inflammatory cytokines (TNF-α, IFN-β), and inhibiting ferroptosis via ubiquitination-mediated downregulation of the POR-ACSL4-LPCAT3 pathway, thereby reducing polyunsaturated fatty acid peroxidation. These synergistic actions significantly improved survival rates and mitigated neuroinflammation. Our findings highlight CeO2/3TT@NP-RVG as a pioneering theranostic platform that bridges molecular mechanism-based therapy with precision imaging, offering a transformative strategy for encephalitis and related neurological disorders.
82
News (Medical) associated with The Chinese University of Hong Kong11 Nov 2025
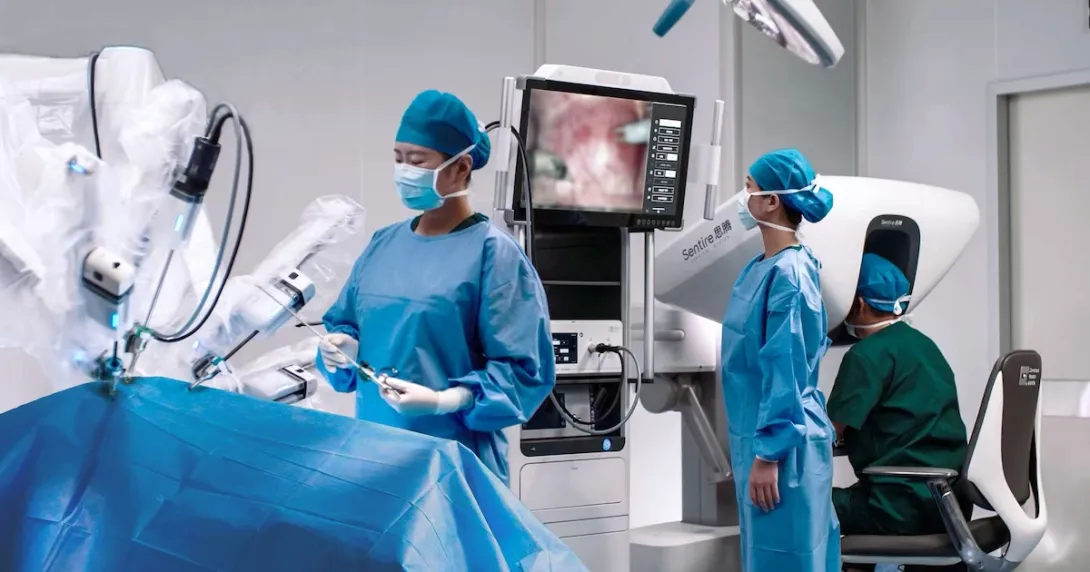
Hong Kong-based surgical robotics maker Cornerstone Robotics has raked in $200 million in funding from a round joined by the Hong Kong Investment Corporation, the Hong Kong government's investment arm.
Existing investors Qiming Venture Partners, Daohe Investment, Gaorong Capital, and ZhenFund also participated in the round.
WHAT'S IT FOR
In a press release, the company – now considered a unicorn startup with a valuation reaching over $1 billion – said it will use its fresh funds to speed up its commercialisation in China and abroad. It will also be infused into the development and regulatory registrations for its upcoming products.
It last raised
$70 million in a Series C round
led by EQT in January.
WHY IT MATTERS
Cornerstone Robotics said it highly values HKIC's investment in the company, viewing it as an endorsement of its capabilities. "Cornerstone Robotics has received continued and strong support from HKIC, including its lead in this round. This not only serves as an authoritative endorsement of our product innovation and global commercialisation capabilities, but also reflects the firm confidence and long-term commitment of Hong Kong's local capital, and even the government, in nurturing technology unicorns," it said in a statement in Chinese.
Founded in 2019, the unicorn startup offers a laparoscopic surgical robot that has been approved and marketed across China and Europe.
MARKET SNAPSHOT
This year also saw Cornerstone Robotics partnering with major health institutions in Hong Kong and Singapore. It is collaborating with the Chinese University of Hong Kong Faculty of Medicine and Singapore's
NHG Health
on projects related to the training, teaching, research, and clinical application of its robotic surgical system.
A similar company in Israel,
ForSight Robotics
, scored $125 million in a Series B funding round in June. It develops a robotic system assisting with ophthalmic surgery.
Meanwhile, another surgical robotics maker from India,
SS Innovations
, recently uplisted on the NASDAQ while expanding into Europe, Asia, and the United States.
08 Nov 2025
Scientists found that DNA’s phosphate groups can direct chemical reactions to make the correct mirror-image form of drug molecules. This breakthrough simplifies chiral drug production, reducing waste and energy use. Using a new “PS scanning” method, the team pinpointed which DNA parts guide reactions. The approach could revolutionize green chemistry in pharmaceuticals.
Researchers at the National University of Singapore (NUS) have uncovered a new way to use deoxyribonucleic acid (DNA). Beyond carrying genetic information, DNA can also serve as a tool for creating medicines more efficiently. Specific regions of DNA known as phosphates act like tiny "hands" that guide chemical reactions to form the correct mirror-image version of a compound.
Many medicines are chiral, meaning they exist in two mirror-image forms -- similar to a pair of hands -- that can behave differently inside the body. One version may effectively treat disease, while the other could have little benefit or even cause harm. Producing only the desired form is a major challenge in drug development, but the new DNA-guided method could make this process cleaner, simpler, and more environmentally sustainable.
DNA and proteins naturally attract one another in living cells because DNA's phosphate groups carry a negative charge, while many amino acids are positively charged. The NUS team, led by Assistant Professor Zhu Ru-Yi from the Department of Chemistry, wondered whether this same type of attraction could help control chemical reactions in the lab. Their goal was to see if DNA could guide molecules to react in specific, predictable ways.
How DNA's Phosphates Guide Chemistry
The researchers found that certain phosphate groups in DNA can pull in positively charged molecules during a chemical reaction, helping them align correctly -- much like a magnet drawing a metal bead into place. This process, known as "ion pairing," keeps the reacting molecules close together and oriented just right to produce a single, desired mirror-image product. The team showed that this guiding effect works across several different chemical reactions.
To pinpoint which phosphates were responsible for this guiding ability, the team created a new experimental approach called "PS scanning." They systematically replaced individual phosphate sites in the DNA with nearly identical substitutes and repeated their tests. When swapping out a phosphate reduced the selectivity of the reaction, it revealed that the original site played a crucial role. To confirm their findings, they collaborated with Professor Zhang Xinglong from The Chinese University of Hong Kong, who used computer simulations to validate the experimental results.
The work was published in Nature Catalysis on October 31, 2025.
DNA as a Green Chemistry Tool
Asst Prof Zhu explained, "Nature never uses DNA phosphates as catalysts, but we have shown that if designed properly, they can act like artificial enzymes."
He added that the discovery could make chemical manufacturing both more sustainable and more efficient, especially when producing complex, high-value pharmaceuticals.
The team plans to continue exploring how DNA phosphates can be used to design and produce chiral (mirror-image) compounds for next-generation drug development.
07 Oct 2025
PHILADELPHIA--(BUSINESS WIRE)--Virion Therapeutics, LLC, a clinical stage biotechnology company, developing novel T cell-based immunotherapies that utilize checkpoint modifiers, today announced that an abstract highlighting key new clinical data from its VRON-0200 Chronic Hepatitis B virus (HBV) program, has been accepted for oral presentation at the upcoming American Association for the Study of Liver Diseases (AASLD), The Liver Meeting®, in Washington, DC, November 7-10, 2025. In addition, Virion announced an abstract highlighting manufacturing enhancements, developed for its platform technologies and used for the VRON-0200 program, has been accepted for oral presentation at the upcoming Vaccines Summit in Boston, MA, November 13-15, 2025.
The AASLD oral presentation will highlight VRON-0200’s safety, tolerability, and clinical activity, including the first long-term VRON-0200 post-dosing results in chronically HBV-infected patients, from its Phase 1b clinical trial.
“Chronic HBV infection affects close to 260 million persons worldwide and contributes to almost 1 million deaths per year from liver related complications. To date, investigational treatments have failed to restore the immunity needed to control the virus, resulting in viral rebound, which is the 'Achilles heel' of current HBV Functional Cure regimens,” said Dr. Andrew D. Luber, Pharm.D., CEO of Virion. Luber added, “We look forward to sharing our latest findings at AASLD, including long-term follow-up responses that support VRON-0200’s potential to be the foundational backbone, as the 'Spark' to a Functional Cure for Chronic HBV.”
Oral Presentation, AASLD’s, The Liver Meeting®:
Title : HBsAg declines observed with VRON-0200 alone are rapidly enhanced with the addition of combination antiviral therapies: results from a Phase 1b study for functional cure in chronically HBV-infected patients (Abstract # 0196) Presenter: Grace Lai-Hung Wong, MD, MBChB, Medical Data Analytics Centre, Department of Medicine and Therapeutics, and Institute of Digestive Disease, The Chinese University of Hong Kong, Hong Kong Session : Next-generation HBV Therapeutics: Emerging Therapies and Search for Functional Cure Date : Sunday, November 9, 2025 Presentation Time : 5:15 pm – 5:30 pm
Oral Presentation, Vaccines Summit:
Title : Application of a digital droplet polymerase chain reaction (ddPCR) assay platform for assessing physical and infectious titers of VRON-0200, a novel therapeutic vaccine Presenter: Paula, MacDonald, Chief Technology Officer, Virion Therapeutics, LLC Session : Bioprocessing & Manufacturing Dates : November 13-15, 2025
About Chronic Hepatitis B
Despite a preventative vaccine, cases of chronic hepatitis B (HBV) continue to rise, with an estimated 254 million persons infected worldwide and 1.1 million deaths per year from HBV-related liver complications. Chronic HBV remains a global health issue with a high unmet medical need, since there is no cure available. The current standard of care requires lifelong antiviral therapy to maintain control of the virus.
About VRON-0200
VRON-0200 is a therapeutic immunotherapy designed with the goal of providing a functional cure for chronic HBV infection. Clinical data from an ongoing Phase 1b trial have shown VRON-0200 to be safe and well tolerated, and, when given as a single intramuscular dose, was immunogenic, and able to “Spark” anti-HBV activity in chronically HBV-infected patients on nucleos(t)ide therapy alone, and, also, when administered first and given with combination antiviral therapies. These results suggest the potential of VRON-0200 to be the key backbone agent, in combination HBV functional cure regimens.
About Virion Therapeutics (Virion)
Virion Therapeutics, LLC is a clinical-stage company developing novel immunotherapies that utilize proprietary checkpoint modifiers to enhance/restore, broaden, and elicit sustained immune responses, with the goal to cure cancer and chronic infectious diseases. Virion has since developed a robust pipeline, including its lead VRON-0200 clinical program, and several additional IND-enabling programs, such as its VRON-0300 oncology program for advanced solid tumors, leveraging its proprietary platform technologies.
To learn more, visit www.VirionTx.com.
VaccinePhase 1ImmunotherapyClinical ResultPhase 2
100 Deals associated with The Chinese University of Hong Kong
Login to view more data
100 Translational Medicine associated with The Chinese University of Hong Kong
Login to view more data
Corporation Tree
Boost your research with our corporation tree data.
login
or

Pipeline
Pipeline Snapshot as of 28 Feb 2026
The statistics for drugs in the Pipeline is the current organization and its subsidiaries are counted as organizations,Early Phase 1 is incorporated into Phase 1, Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3
Discovery
10
15
Preclinical
Phase 1
1
3
Phase 2
Other
10
Login to view more data
Current Projects
| Drug(Targets) | Indications | Global Highest Phase |
|---|---|---|
Erzhi Pills | Hypercholesterolemia More | Phase 2 |
Renzhu Changle Granules | Irritable bowel syndrome with diarrhea More | Phase 2 |
Autologous mesenchymal stem cell therapy(The Chinese University of Hong Kong) | Osteoarthritis, Knee More | Phase 1 |
Dorzagliatin ( glucokinase ) | Hyperglycemia More | Clinical |
SIM-01 | Post Acute COVID 19 Syndrome More | Clinical |
Login to view more data
Deal
Boost your decision using our deal data.
login
or

Translational Medicine
Boost your research with our translational medicine data.
login
or

Profit
Explore the financial positions of over 360K organizations with Synapse.
login
or

Grant & Funding(NIH)
Access more than 2 million grant and funding information to elevate your research journey.
login
or

Investment
Gain insights on the latest company investments from start-ups to established corporations.
login
or

Financing
Unearth financing trends to validate and advance investment opportunities.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free


