A Comprehensive Review of Cinacalcet hydrochloride's R&D Innovations
Cinacalcet hydrochloride's R&D Progress
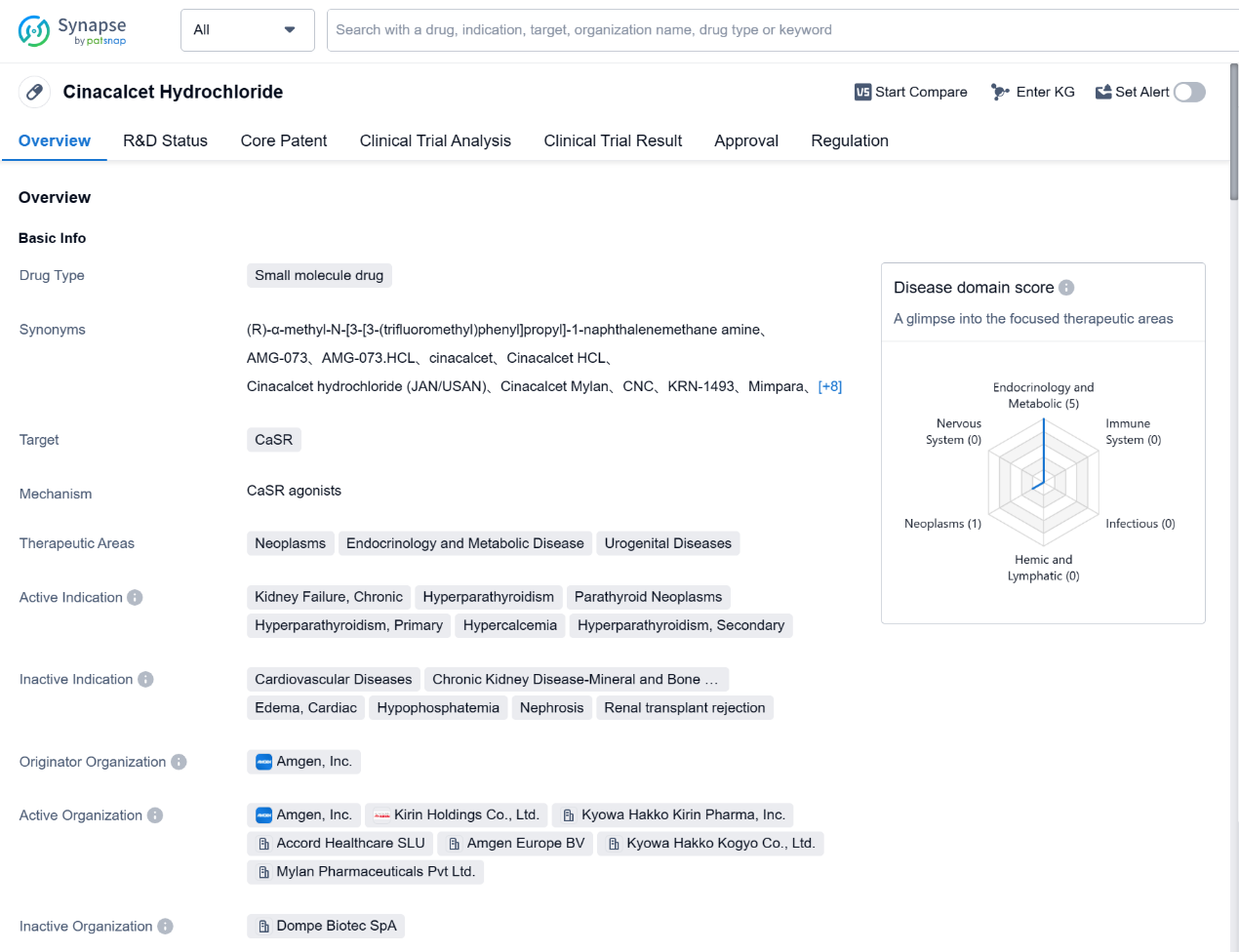
Cinacalcet Hydrochloride is a small molecule drug that targets the Calcium Sensing Receptor (CaSR). It is primarily used in the treatment of various conditions related to neoplasms, endocrinology and metabolic diseases, and urogenital diseases. The drug has been approved for use in the treatment of kidney failure, chronic hyperparathyroidism, parathyroid neoplasms, hyperparathyroidism (primary and secondary), and hypercalcemia.
The originator organization of Cinacalcet Hydrochloride is Amgen, Inc. The drug has received approval in both the global market and China. Its first approval date in the global market was in March 2004, with the United States being the first country/location to approve its use.
Cinacalcet Hydrochloride has undergone priority review and has also been designated as an orphan drug. Priority review is a regulatory process that expedites the review of drugs that offer significant improvements in the treatment, diagnosis, or prevention of serious conditions. Orphan drug designation is granted to drugs that are intended to treat rare diseases or conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Cinacalcet hydrochloride: CaSR agonists
CaSR agonists are a type of drugs that activate the Calcium-sensing receptor (CaSR). The CaSR is a G-protein coupled receptor found in various tissues, including the parathyroid glands, kidneys, and bones. It plays a crucial role in regulating calcium homeostasis in the body.
From a biomedical perspective, CaSR agonists are used in the treatment of conditions related to abnormal calcium levels, such as hyperparathyroidism and hypocalcemia. By activating the CaSR, these drugs help to regulate the release of parathyroid hormone and control calcium levels in the blood.
CaSR agonists can have different mechanisms of action depending on the specific drug. Some may directly bind to the CaSR and activate it, while others may modulate the receptor indirectly. By targeting the CaSR, these drugs can help restore normal calcium balance and alleviate symptoms associated with calcium imbalances.
Drug Target R&D Trends for Cinacalcet hydrochloride
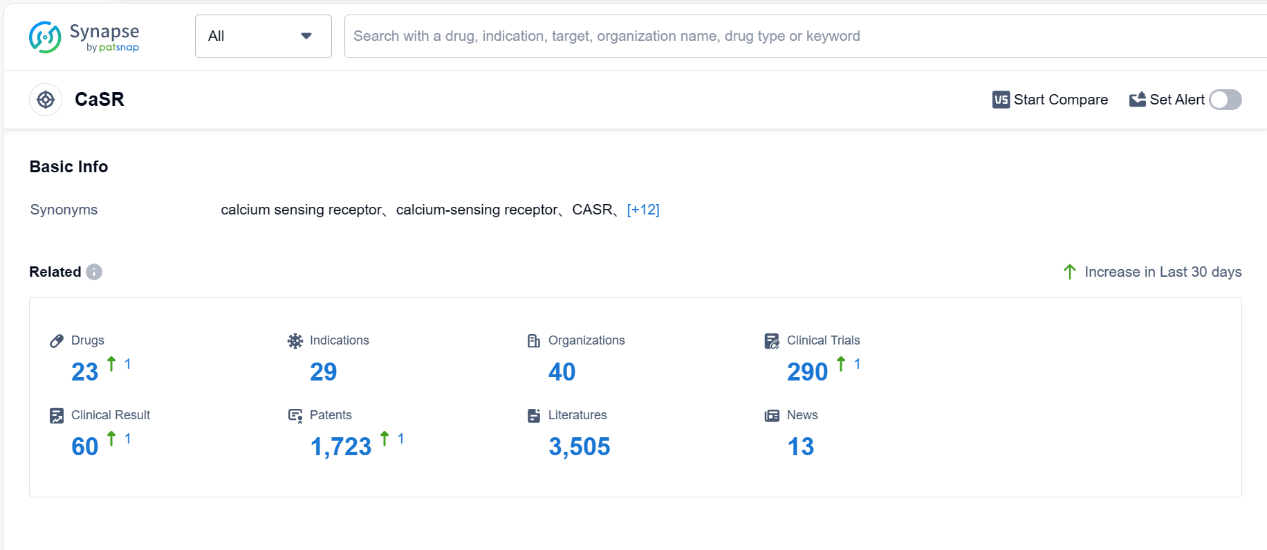
The Calcium Sensing Receptor (CaSR) is a protein found in various tissues of the human body, including the parathyroid glands, kidneys, and gastrointestinal tract. It plays a crucial role in maintaining calcium homeostasis by sensing changes in extracellular calcium levels. When activated by high calcium levels, CaSR inhibits the release of parathyroid hormone, which helps regulate calcium levels in the blood. Additionally, CaSR is involved in the regulation of renal calcium reabsorption and intestinal calcium absorption. Dysfunction of CaSR can lead to disorders such as hypercalcemia or hypocalcemia, highlighting its importance in maintaining calcium balance and overall health. According to PatSnap Synapse, as of 4 Sep 2023, there are a total of 23 CaSR drugs worldwide, from 40 organizations, covering 29 indications, and conducting 290 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Cinacalcet Hydrochloride is a small molecule drug developed by Amgen, Inc. It targets the CaSR and is used in the treatment of various conditions related to neoplasms, endocrinology and metabolic diseases, and urogenital diseases. It has been approved for use in the treatment of kidney failure, chronic hyperparathyroidism, parathyroid neoplasms, hyperparathyroidism (primary and secondary), and hypercalcemia. The drug has received global and China approvals, with its first approval in the United States in March 2004. It has undergone priority review and has been designated as an orphan drug.