Brief Discussion on Clinically Under-Investigation BRD4 Inhibitors
Bromodomain (BRDs) is a conserved protein structural domain that can specifically recognize acetylated lysine residues in proteins. Based on their structure and sequence similarity, 61 human bromodomains are classified into eight families, among which the BET family proteins, including BRD2, BRD3, BRD4, and BRDT are the most representative. The BET family bromodomain protein BRD4 contains acetylated lysine residues capable of binding chromatin and other proteins, which play a crucial role in gene regulation and cell growth control. BRD4 is associated with large protein complexes involved in regulating gene transcription, including mediator, PAFc, and super elongation complex, etc. The kinase activity of BRD4 can directly phosphorylate and activate RNA polymerase II, thereby regulating gene transcription expression.
Many human diseases are closely related to BRD4 protein, such as tumors, autoimmune or inflammatory diseases, viral infections, and so on. BRD4 inhibitors target BRD4 for inhibition, which hold tremendous value in anti-cancer and anti-inflammatory sectors, as well as many other fields. It continues to attract the attention of major pharmaceutical companies and research institutions.
BRD4 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 8 Sep 2023, there are a total of 76 BRD4 drugs worldwide, from 62 organizations, covering 57 indications, and conducting 58 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.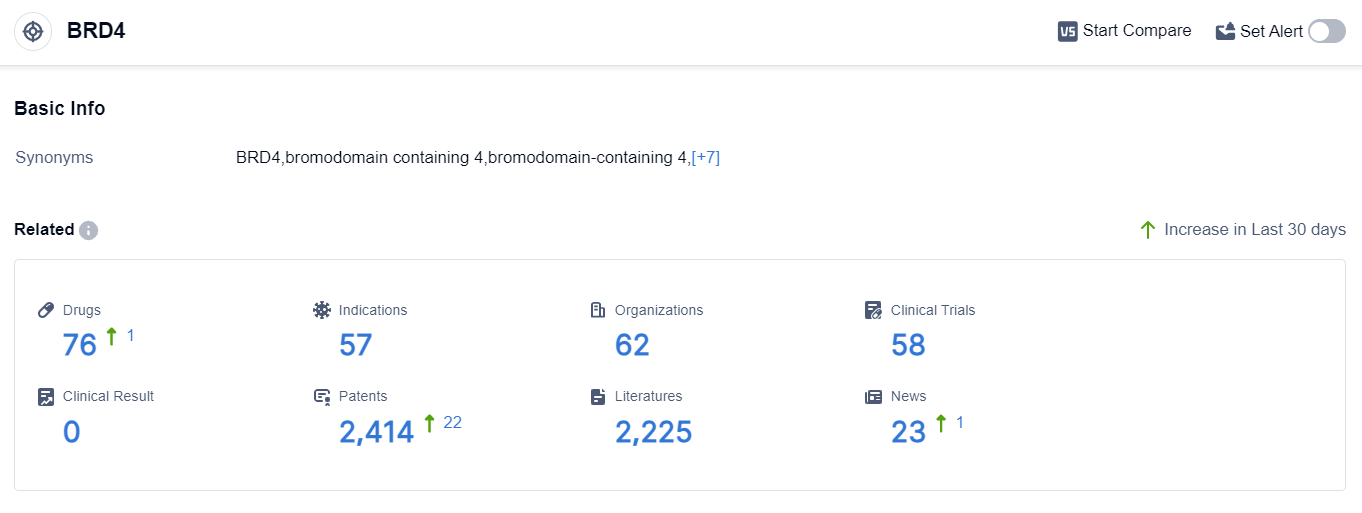
The analysis of the target BRD4 reveals a competitive landscape with multiple companies actively involved in its development. Resverlogix Corp., Shenzhen Hepalink Pharmaceutical Group Co., Ltd., and Daiichi Sankyo Co., Ltd. are among the companies showing significant progress in their R&D efforts.
The approved indications for drugs targeting BRD4 cover a wide range of diseases, indicating its potential as a therapeutic target. Small molecule drugs and PROTACs are the drug types progressing most rapidly, suggesting intense competition in the market.
The countries/locations with active development include the United States, European Union, China, and others. China has shown progress in the development of BRD4 drugs.
Overall, the target BRD4 presents a promising opportunity for pharmaceutical companies, with a diverse range of indications and active research and development efforts worldwide.
BRD4 Inhibitor in Phase III Clinical Trials: Apabetalone
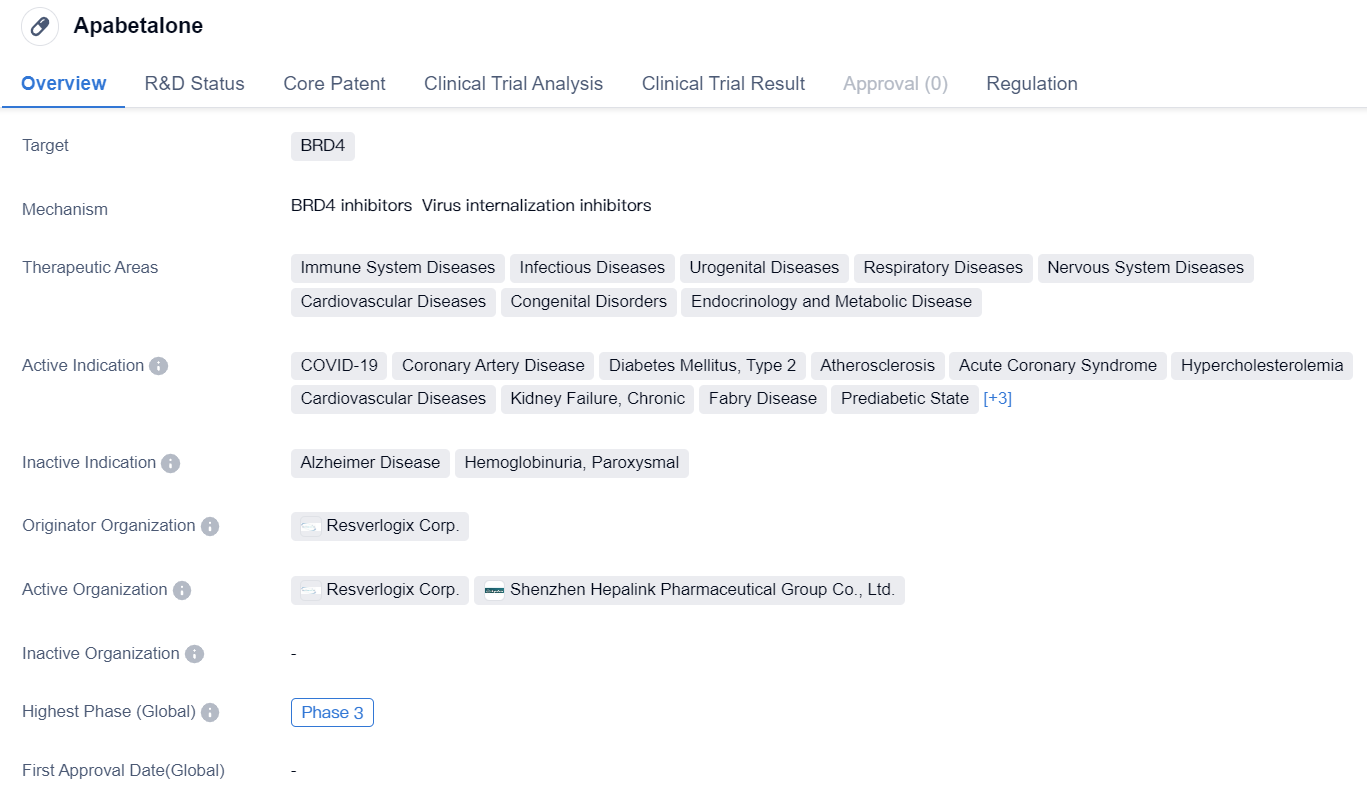
Apabetalone is a small molecule drug that targets BRD4, a protein involved in various cellular processes. It has shown potential therapeutic benefits in a wide range of therapeutic areas, including immune system diseases, infectious diseases, urogenital diseases, respiratory diseases, nervous system diseases, cardiovascular diseases, congenital disorders, endocrinology, and metabolic diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In terms of active indications, Apabetalone has demonstrated efficacy in COVID-19, coronary artery disease, type 2 diabetes mellitus, atherosclerosis, acute coronary syndrome, hypercholesterolemia, kidney failure, chronic, Fabry disease, prediabetic state, diabetic nephropathies, pulmonary arterial hypertension, and HIV infections. This suggests that the drug may have a broad spectrum of applications in treating various diseases and conditions.
Apabetalone is developed by Resverlogix Corp., an originator organization specializing in the pharmaceutical industry. Currently, the drug is currently in Phase 3 of clinical development. This indicates that it has progressed through earlier stages of testing and is now being evaluated in larger populations to assess its safety and efficacy.
Furthermore, Apabetalone has been granted Breakthrough Therapy designation, a regulatory status that recognizes its potential to provide significant benefits over existing treatments for serious or life-threatening conditions. This designation highlights the drug's potential to address unmet medical needs and expedite its development and review process.
In summary, Apabetalone is a small molecule drug that targets BRD4 and has shown promise in various therapeutic areas, including immune system diseases, infectious diseases, urogenital diseases, respiratory diseases, nervous system diseases, cardiovascular diseases, congenital disorders, endocrinology, and metabolic diseases. It is currently in Phase 3 of development and has been granted Breakthrough Therapy designation, indicating its potential to address unmet medical needs.
BRD4 inhibitor entering Phase II Clinical Trials: NHWD-870
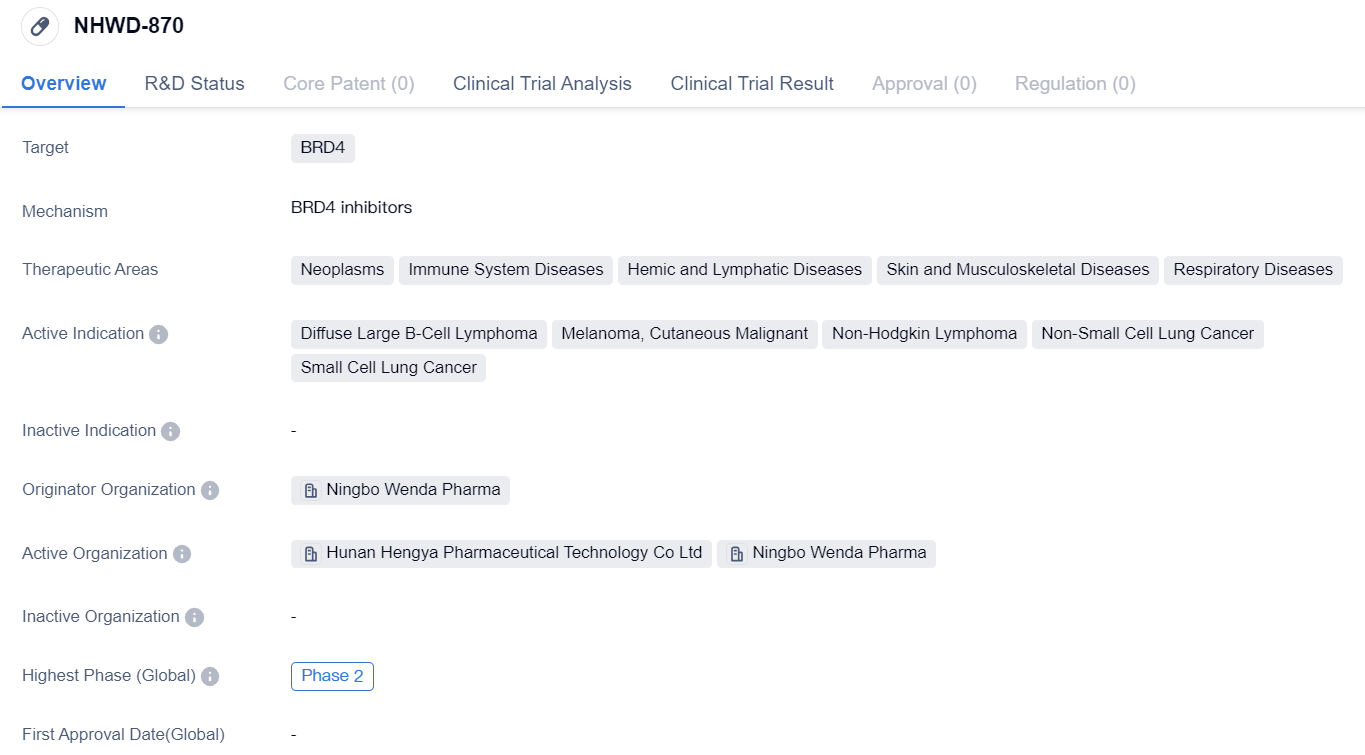
NHWD-870 is a small molecule drug developed by Ningbo Wenda Pharma. It is designed to target BRD4, a protein that plays a role in various diseases. The drug is currently in Phase 2 of clinical trials, both globally and in China.
The therapeutic areas that NHWD-870 aims to address include neoplasms (abnormal growth of cells), immune system diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, and respiratory diseases. This indicates that the drug has potential applications in a wide range of medical conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The active indications for NHWD-870 are diffuse large B-cell lymphoma, melanoma (cutaneous malignant), non-Hodgkin lymphoma, non-small cell lung cancer, and small cell lung cancer. These are all serious and life-threatening diseases, highlighting the importance of developing effective treatments in these areas.
Ningbo Wenda Pharma is the originator organization behind NHWD-870. As the developer of the drug, they are responsible for its research, development, and clinical trials. The fact that NHWD-870 has reached Phase 2 in both global and Chinese trials suggests that it has shown promising results in earlier stages of testing.
Overall, NHWD-870 is a small molecule drug that targets BRD4 and has potential applications in various therapeutic areas, including neoplasms, immune system diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, and respiratory diseases. Its active indications include diffuse large B-cell lymphoma, melanoma, non-Hodgkin lymphoma, non-small cell lung cancer, and small cell lung cancer. Developed by Ningbo Wenda Pharma, NHWD-870 has reached Phase 2 in clinical trials, indicating its potential as a treatment option for these serious medical conditions.