Exploring Voclosporin's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Voclosporin's R&D Progress
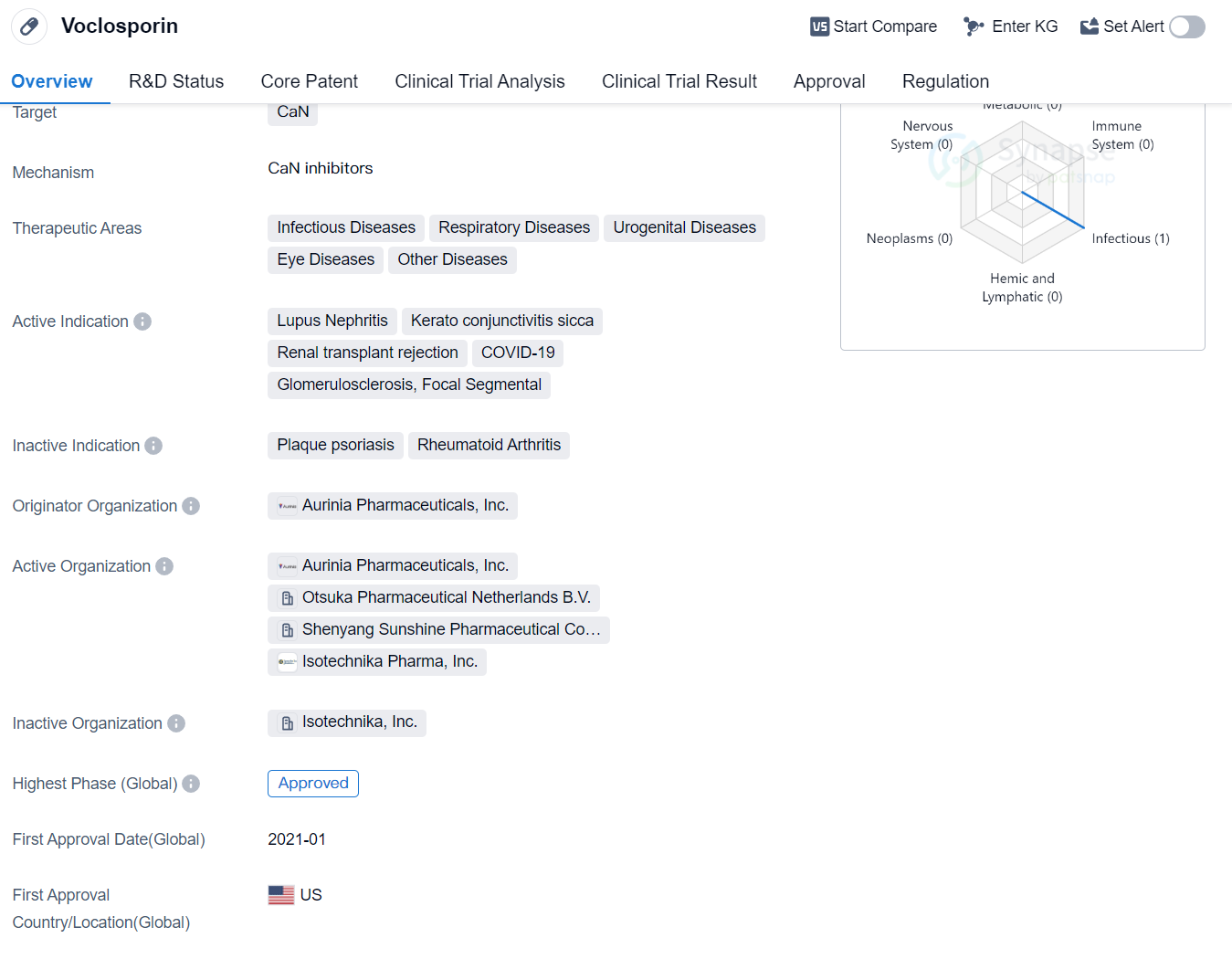
Voclosporin is a synthetic peptide drug that targets CaN (calcineurin), a protein involved in various cellular processes. It has shown potential therapeutic benefits in multiple therapeutic areas, including infectious diseases, respiratory diseases, urogenital diseases, eye diseases, and other diseases.
The drug has been specifically indicated for the treatment of Lupus Nephritis, a condition characterized by inflammation of the kidneys in patients with systemic lupus erythematosus. It has also shown promise in treating Keratoconjunctivitis Sicca, a chronic dry eye condition, and renal transplant rejection, where the body's immune system attacks the transplanted organ.
In addition to these indications, Voclosporin has also been explored as a potential treatment for COVID-19, the respiratory illness caused by the novel coronavirus. This suggests that the drug may have antiviral properties or could help modulate the immune response associated with the disease. Furthermore, it has shown potential in treating Glomerulosclerosis, a condition characterized by scarring of the kidney's filtering units, and Focal Segmental Glomerulosclerosis, a specific subtype of glomerulosclerosis.
Voclosporin was developed by Aurinia Pharmaceuticals, Inc., an originator organization specializing in the pharmaceutical industry. The drug has reached the highest phase of development, with global approval obtained in January 2021. The United States was the first country to approve Voclosporin for commercial use.
In terms of regulatory status, Voclosporin has been granted Fast Track designation, which expedites the development and review process for drugs that address unmet medical needs. Additionally, it has received Orphan Drug designation, which provides incentives to encourage the development of drugs for rare diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Voclosporin: CaN inhibitor
CaN inhibitors refer to a class of compounds that inhibit the activity of the enzyme calcineurin (CaN). Calcineurin is a protein phosphatase that plays a crucial role in many cellular processes, including immune response, neuronal signaling, and cardiac function. By inhibiting CaN, these inhibitors can modulate the activity of various signaling pathways and have potential therapeutic applications.
From a biomedical perspective, CaN inhibitors are of particular interest in the field of immunology. They can suppress the immune response by inhibiting the activation of T cells, which are crucial for the immune system's proper functioning. This immunosuppressive effect makes CaN inhibitors valuable in the treatment of autoimmune diseases, organ transplantation, and certain inflammatory conditions.
CaN inhibitors can be classified into two main types: cyclosporine A (CsA) and tacrolimus (FK506). These drugs bind to specific proteins called immunophilins, forming complexes that inhibit the activity of calcineurin. By doing so, they prevent the dephosphorylation of nuclear factor of activated T cells (NFAT), a transcription factor that regulates the expression of genes involved in immune response. This ultimately leads to the suppression of T-cell activation and the dampening of immune reactions.
It is important to note that the use of CaN inhibitors requires careful monitoring and management due to their potential side effects, such as increased susceptibility to infections and the risk of organ toxicity.
Drug Target R&D Trends for Voclosporin
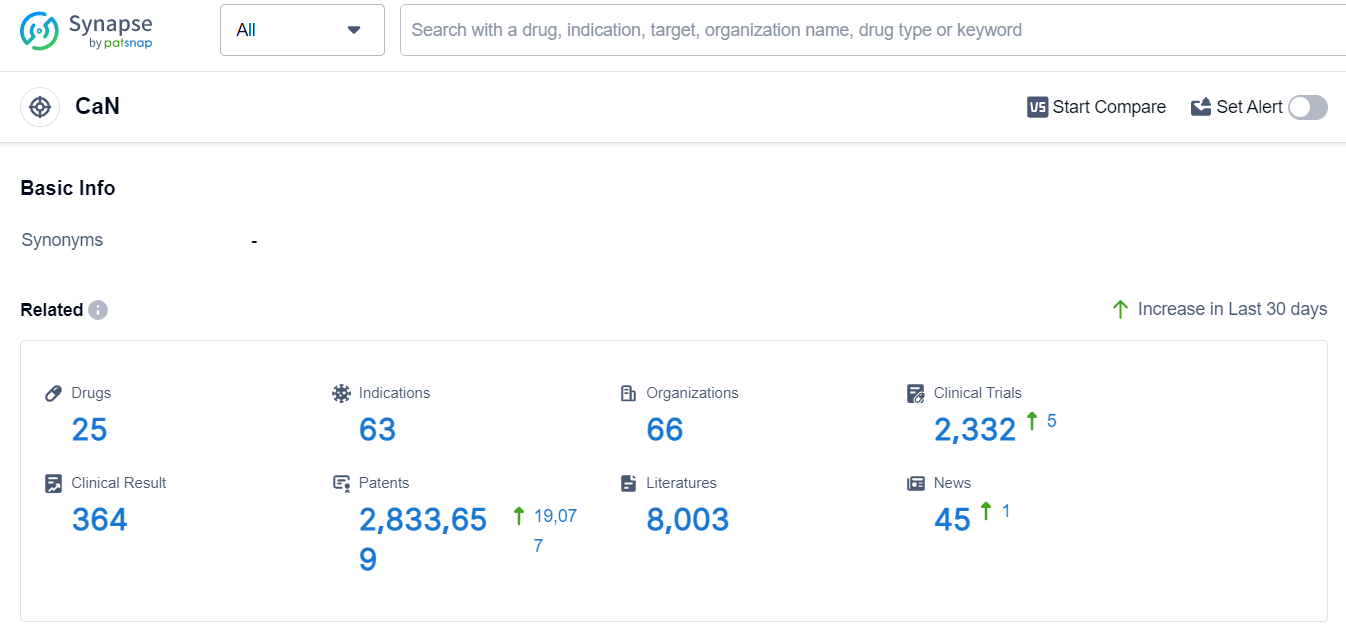
The analysis of the current competitive landscape for target CaN reveals that Novartis AG, Astellas Pharma, Inc., Aurinia Pharmaceuticals, Inc., and Huons Co., Ltd. are the companies growing fastest in this field. These companies have made significant progress in the R&D of drugs targeting CaN. The indications for which drugs have been approved under the target CaN cover a wide range of conditions, indicating the potential therapeutic applications of these drugs. Small molecule drugs and synthetic peptides are the drug types progressing most rapidly, suggesting intense competition, especially in the case of biosimilars. The United States, European Union, and China are the countries/locations developing fastest under the target CaN, with China showing significant progress. Overall, the target CaN presents a competitive landscape with multiple companies, diverse indications, and various drug types, indicating a promising future for the development of drugs targeting this pathway.
According to Patsnap Synapse, as of 6 Sep 2023, there are a total of 25 CaN drugs worldwide, from 66 organizations, covering 63 indications, and conducting 2332 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Voclosporin is a synthetic peptide drug that targets CaN and has shown potential in various therapeutic areas, including lupus nephritis, keratoconjunctivitis sicca, renal transplant rejection, COVID-19, glomerulosclerosis, and focal segmental glomerulosclerosis. Its approval in the United States and regulatory designations highlight its potential as a treatment option for patients with these conditions.