Decoding Vismodegib: A Comprehensive Study of its R&D Trends
Vismodegib's R&D Progress
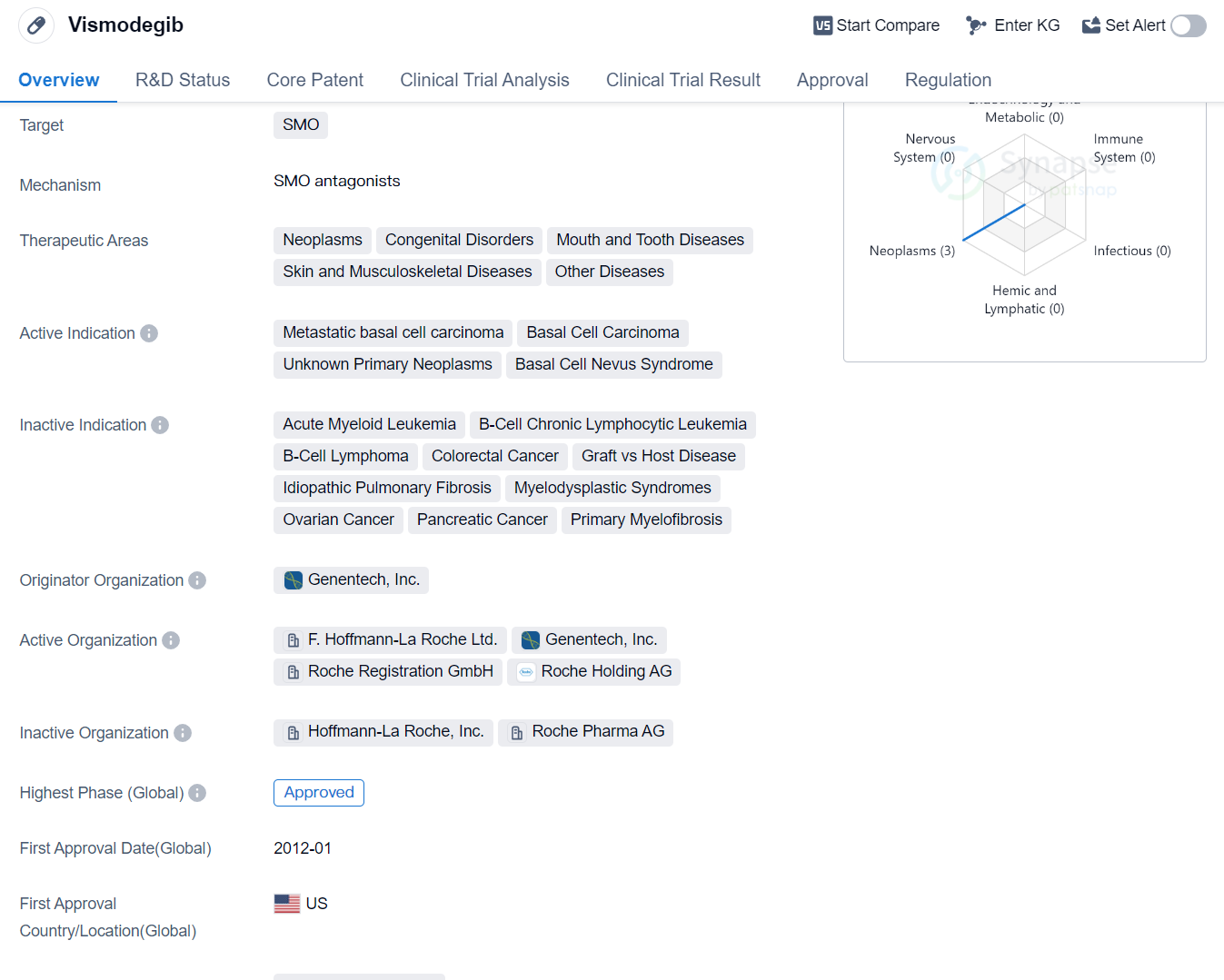
Vismodegib is a small molecule drug that falls under the category of biomedicine. It primarily targets the SMO protein. The drug has shown potential in treating various therapeutic areas including neoplasms, congenital disorders, mouth and tooth diseases, skin and musculoskeletal diseases, and other diseases.
Vismodegib has been specifically approved for the treatment of metastatic basal cell carcinoma, basal cell carcinoma, unknown primary neoplasms, and basal cell nevus syndrome. The drug was first approved in the United States in January 2012. It is important to note that Vismodegib has reached the highest phase of development, which is the approved stage.
The originator organization of Vismodegib is Genentech, Inc., a renowned pharmaceutical company. The drug has gained significant recognition and approval in the global market. It is classified as an overseas new drug urgently needed in clinical settings, indicating its potential to address critical medical needs.
Vismodegib's approval in the United States in 2012 highlights its effectiveness in treating metastatic basal cell carcinoma and other related conditions. The drug's small molecule nature allows it to target the SMO protein, which plays a crucial role in the development and progression of various diseases.
The therapeutic areas targeted by Vismodegib indicate its potential to address a wide range of medical conditions. Neoplasms, congenital disorders, mouth and tooth diseases, and skin and musculoskeletal diseases are among the areas where Vismodegib has shown promise.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Vismodegib: SMO antagonist
SMO antagonists are a type of drugs that act on the Smoothened (SMO) protein, which is involved in the Hedgehog signaling pathway. The Hedgehog pathway plays a crucial role in embryonic development and tissue homeostasis. However, aberrant activation of this pathway has been linked to the development and progression of various cancers, including basal cell carcinoma and medulloblastoma.
SMO antagonists work by inhibiting the activity of the SMO protein, thereby blocking the Hedgehog signaling pathway. By doing so, they help to prevent the uncontrolled growth and proliferation of cancer cells that rely on this pathway for survival. These drugs are considered targeted therapies as they specifically target a molecular component involved in the development of certain cancers.
In the context of biomedicine, SMO antagonists have shown promising results in the treatment of cancers associated with abnormal Hedgehog signaling. They can be used as a therapeutic option either alone or in combination with other anticancer drugs, depending on the specific cancer type and stage. However, it is important to note that like any other medication, SMO antagonists may have side effects and should be used under the supervision of a healthcare professional.
Drug Target R&D Trends for Vismodegib
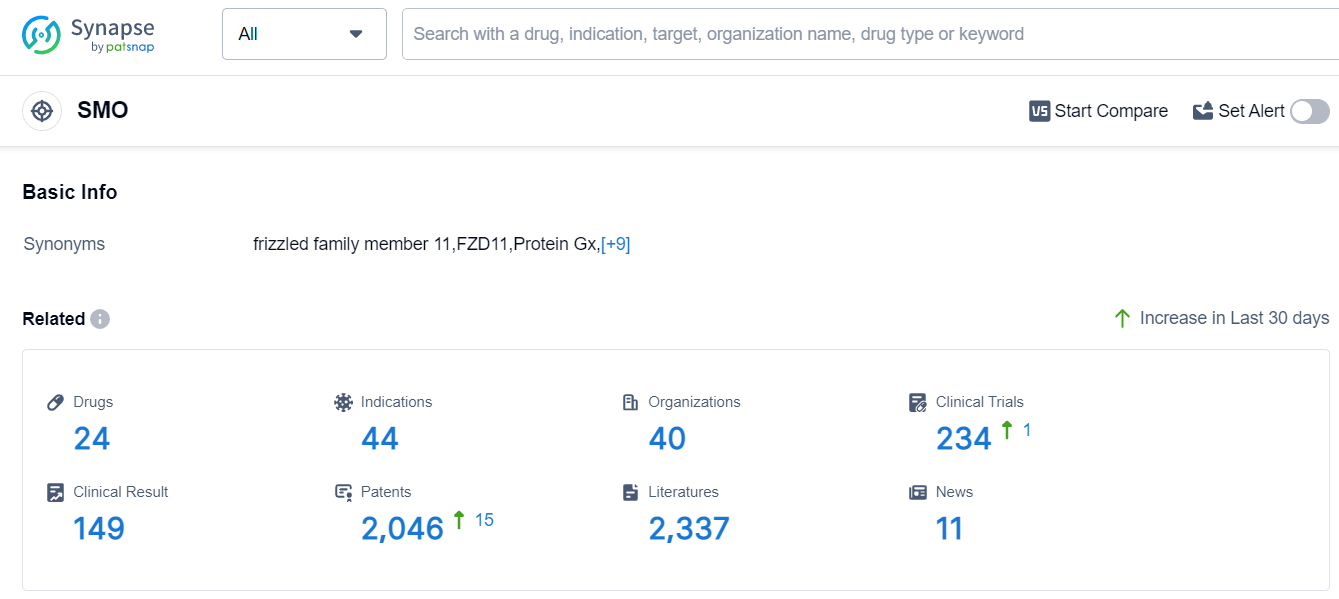
The analysis of the target SMO reveals a competitive landscape with multiple companies experiencing growth. Pfizer Inc., Novartis AG, and Roche Holding AG are among the companies with the highest number of drugs in various development phases. Drugs have been approved for indications such as Basal Cell Carcinoma, Acute Myeloid Leukemia, and Adult Acute Myeloblastic Leukemia. Small molecule drugs and chemical drugs are progressing rapidly, indicating intense competition in the development of innovative drugs. The countries/locations actively involved in drug development include the United States, European Union, Canada, Australia, and China. Further analysis is required to determine the extent of progress in China. Overall, the target SMO presents a competitive landscape with promising future development opportunities.
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 24 SMO drugs worldwide, from 40 organizations, covering 44 indications, and conducting 234 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Vismodegib's approval and its target indication for metastatic basal cell carcinoma and other related conditions make it a significant drug in the field of biomedicine. Its originator organization, Genentech, Inc., adds credibility to its development and potential impact in the pharmaceutical industry. As an overseas new drug urgently needed in clinical settings, Vismodegib holds promise for patients suffering from various diseases, providing a potential breakthrough in their treatment options.