A Comprehensive Review of Pemigatinib's R&D Innovations and Drug Target Mechanism
Pemigatinib's R&D Progress
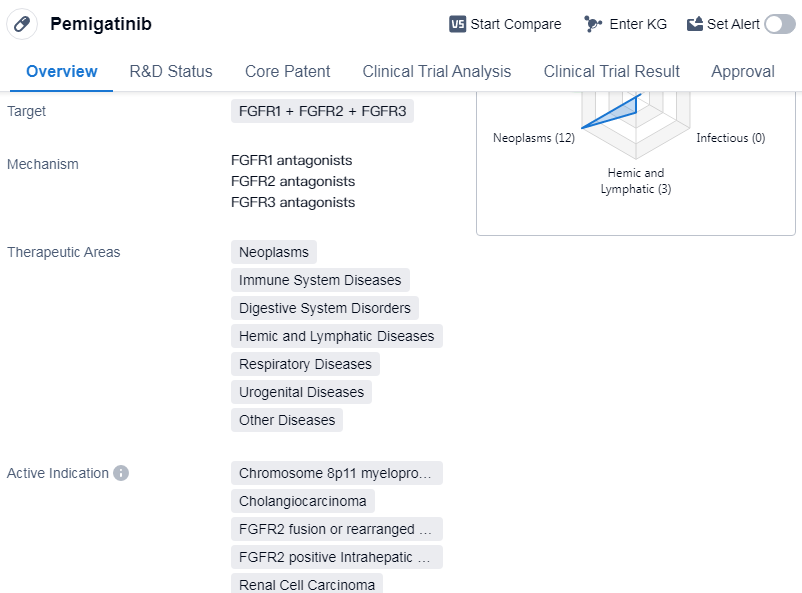
Pemigatinib is a small molecule drug that targets FGFR1, FGFR2, and FGFR3. It has been developed by Incyte Corp. and has received approval for use in several therapeutic areas, including neoplasms, immune system diseases, digestive system disorders, hemic and lymphatic diseases, respiratory diseases, and other diseases.
Pemigatinib has shown promising results in the treatment of various indications. It has been approved for the treatment of colorectal cancer, bladder cancer, transitional cell carcinoma, myeloproliferative disorders, lymphoma, etc.
Pemigatinib has achieved the highest phase of approval globally. It received its first approval in the United States in April 2020. The drug has undergone regulatory processes such as priority review, accelerated approval, orphan drug designation, and breakthrough therapy designation.
The approval of pemigatinib marks a significant advancement in the field of biomedicine. Its ability to target multiple FGFRs makes it a promising option for the treatment of various diseases. The drug's approval for different indications demonstrates its potential in addressing a wide range of medical conditions.
As a small molecule drug, pemigatinib offers advantages such as ease of administration and potential for oral delivery.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pemigatinib: FGFR1 antagonists, FGFR2 antagonists, and FGFR3 antagonists
FGFR1 antagonists, FGFR2 antagonists, and FGFR3 antagonists refer to a group of compounds or drugs that specifically target and inhibit the activity of fibroblast growth factor receptor 1 (FGFR1), fibroblast growth factor receptor 2 (FGFR2), and fibroblast growth factor receptor 3 (FGFR3), respectively.
From a biomedical perspective, fibroblast growth factor receptors (FGFRs) are a family of cell surface receptors that play a crucial role in various cellular processes, including cell growth, proliferation, and differentiation. Abnormal activation or overexpression of FGFRs has been implicated in the development and progression of several diseases, particularly cancers and certain genetic disorders.
FGFR1 antagonists, FGFR2 antagonists, and FGFR3 antagonists are designed to specifically bind to the respective FGFR isoforms and inhibit their signaling pathways. By blocking the activation of these receptors, these antagonists can potentially impede the growth and survival of cancer cells or alleviate the symptoms associated with FGFR-related genetic disorders.
It's worth noting that FGFR antagonists can vary in their chemical structures and mechanisms of action. Some may directly bind to the receptor's extracellular domain, preventing the binding of fibroblast growth factors (FGFs) and subsequent receptor activation. Others may interfere with intracellular signaling pathways downstream of the receptor, inhibiting cell proliferation and promoting cell death.
Overall, FGFR1 antagonists, FGFR2 antagonists, and FGFR3 antagonists hold therapeutic potential in the treatment of FGFR-related diseases, particularly cancers and genetic disorders associated with abnormal FGFR signaling.
Drug Target R&D Trends for Pemigatinib
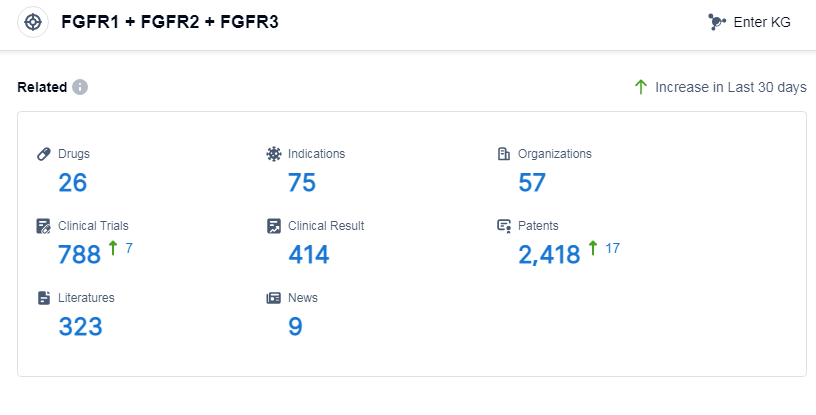
According to Patsnap Synapse, as of 9 Sep 2023, there are a total of 26 FGFR1, FGFR2 and FGFR3 drugs worldwide, from 57 organizations, covering 75 indications, and conducting 788 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of the target FGFR1, FGFR2 and FGFR3 in the pharmaceutical industry is characterized by the involvement of multiple companies, including Eisai Co., Ltd., Johnson & Johnson, BridgeBio Pharma, Inc., etc. These companies have drugs in various stages of development.
Drugs targeting FGFR1, FGFR2 and FGFR3 have been approved for indications such as cholangiocarcinoma, bladder cancer, transitional cell carcinoma, endometrial carcinoma, hepatocellular carcinoma, and others. The highest phase of development for these indications is approved, suggesting successful clinical trials and regulatory approvals.
Small molecule drugs are progressing rapidly under the current targets. The research and development institutions of these biosimilars need further analysis to understand the competitive landscape.
Countries/locations such as the United States, Canada, China, European Union, and Japan are developing fastest under the current targets, with drugs in approved, preclinical, and inactive phases. China's progress with drugs in approved and preclinical phases, highlights its growing presence in the development of drugs targeting FGFR1, FGFR2 and FGFR3.
Overall, the target FGFR1, FGFR2 and FGFR3 shows a promising competitive landscape and future development potential in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Pemigatinib is a small molecule drug developed by Incyte Corp. that targets FGFR1, FGFR2, and FGFR3. It has received approval for the treatment of various indications in multiple therapeutic areas. The drug's approval in the United States in April 2020 and its regulatory designations highlight its potential as an effective treatment option.