AbbVie Reports Positive Phase 3 Results for Tavapadon in Solo Parkinson's Treatment
AbbVie (NYSE: ABBV) has released favorable primary findings from the key Phase 3 TEMPO-1 study assessing Tavapadon as a standalone treatment for early-stage Parkinson’s disease. Tavapadon, a research-stage partial agonist targeting D1/D5 dopamine receptors, is under investigation for its potential as a daily single-dose therapy in Parkinson’s disease management.
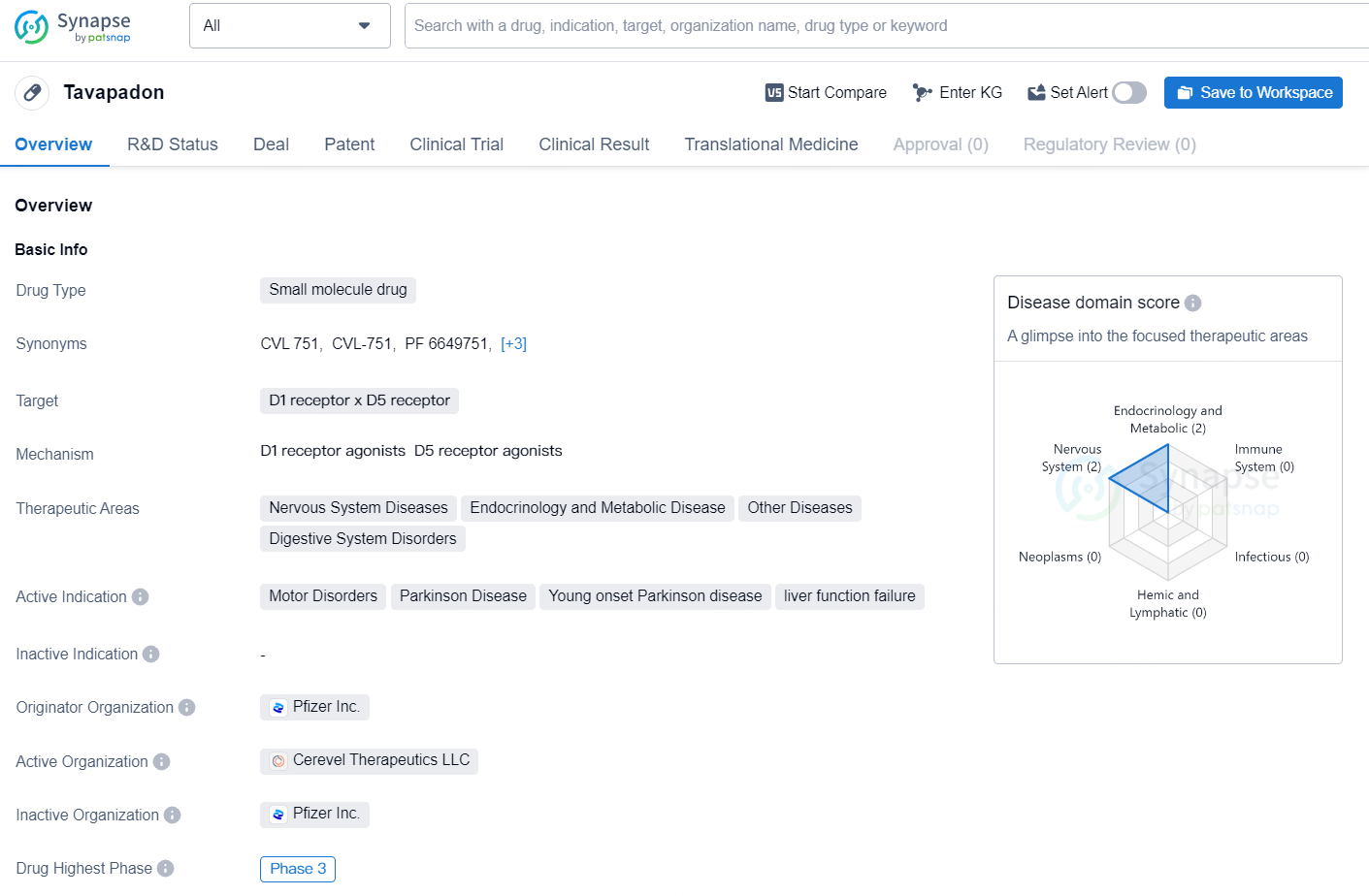
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The TEMPO-1 trial assessed the effectiveness, safety, and tolerability of tavapadon at two fixed doses (5 mg and 15 mg, administered once daily) as a monotherapy for adults in the early stages of Parkinson’s disease. The trial successfully achieved its primary endpoint, demonstrating that patients receiving tavapadon, in both dose groups, showed a statistically significant reduction (improvement) in baseline symptoms compared to those on placebo (placebo: +1.8; 5 mg: -9.7; 15 mg: -10.2; p-value <0.0001 for each dose versus placebo) according to the combined score of the Movement Disorder Society – Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Parts II and III at week 26.
In addition, the TEMPO-1 trial also met its key secondary endpoint by showing a statistically significant and clinically meaningful improvement in the motor aspects of daily living experiences (MDS-UPDRS Part II) in both dose groups of tavapadon compared to placebo by week 26.
"The TEMPO-1 data, along with the previously reported results from the TEMPO-3 adjunctive trial, underscore the potential of tavapadon for individuals with Parkinson’s disease," stated Primal Kaur, MD, MBA, senior vice president of immunology, neuroscience, eye care, and specialty development at AbbVie. "This represents a crucial advancement in our commitment to strengthening our neuroscience portfolio following the strategic acquisition of Cerevel Therapeutics and exemplifies our dedication to supporting patients at every stage of this complex neurological condition. We anticipate sharing further data later this year from the TEMPO-2 monotherapy trial."
The safety profile observed in the TEMPO-1 trial was consistent with prior clinical studies, with most adverse events being mild to moderate in severity.
The complete results from the TEMPO-1 trial will be submitted for presentation at upcoming medical meetings and will be used to support regulatory submissions for tavapadon as a treatment for Parkinson's disease. Preliminary results from TEMPO-2, the Phase 3 flexible-dose monotherapy trial for tavapadon, are expected by the end of 2024.
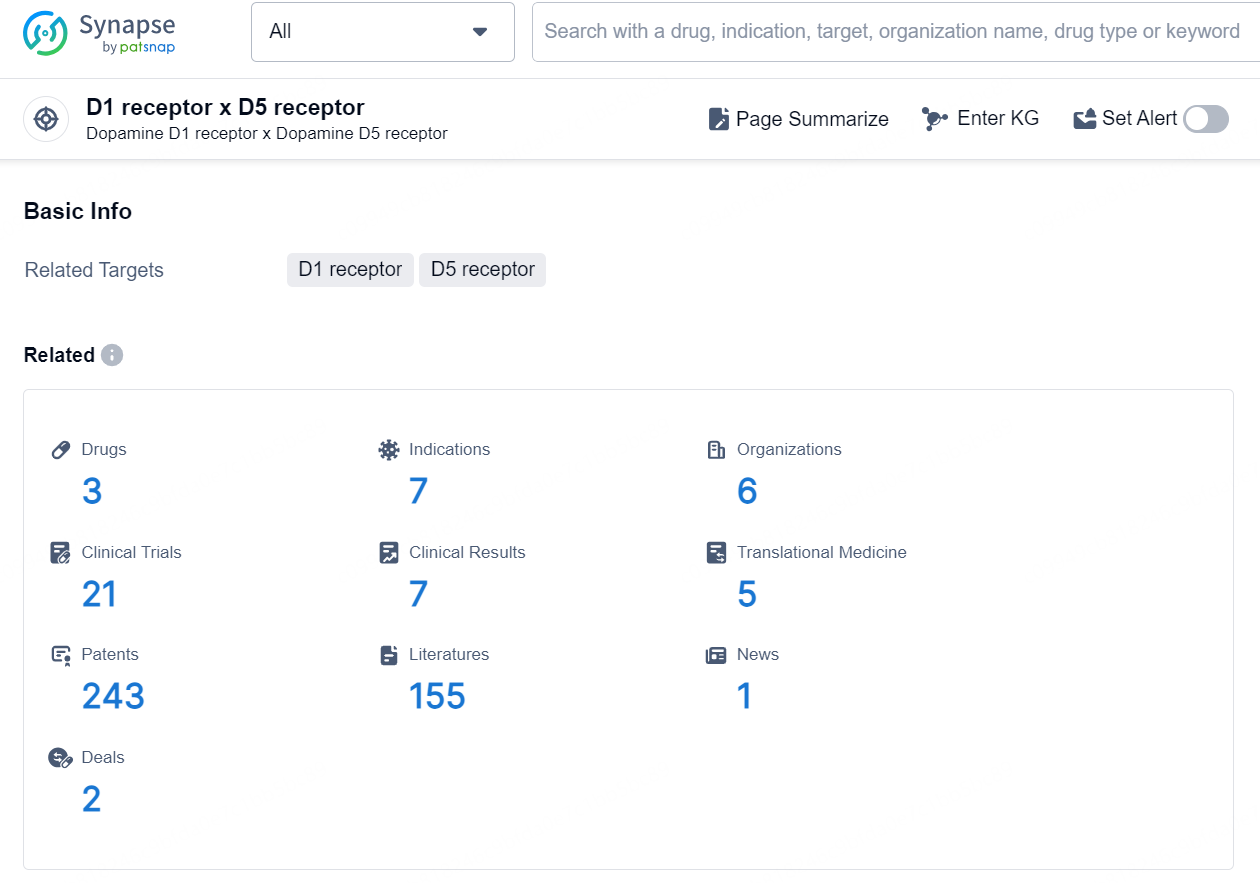
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 30, 2024, there are 3 investigational drug for the D1 receptor x D5 receptor targets, including 7 indications, 6 R&D institutions involved, with related clinical trials reaching 21, and as many as 243 patents.
Tavapadon is a small molecule drug developed by Pfizer Inc., targeting the D1 and D5 receptors. The drug is in the highest phase of global development, with Phase 3 clinical trials underway. The therapeutic areas of Tavapadon include Nervous System Diseases, Endocrinology and Metabolic Disease, Other Diseases, and Digestive System Disorders. The drug is primarily indicated for the treatment of Motor Disorders, Parkinson's Disease, Young-Onset Parkinson's Disease, and liver function failure.