AC Immune Reports Positive Preliminary Results from Phase 2 Study of ACI-7104.056 for Early Parkinson’s Disease
AC Immune SA (NASDAQ: ACIU), a biopharmaceutical firm focused on developing precision therapies for neurodegenerative disorders, has reported favorable interim safety and immunogenicity outcomes from the Phase 2 VacSYn clinical trial assessing ACI-7104.056. This candidate, which is an active immunotherapy targeting anti-alpha-synuclein (a-syn), is intended for use in patients diagnosed with early-stage Parkinson’s disease (PD).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Dr. Andrea Pfeifer, the CEO of AC Immune SA, stated, “We are pleased with the preliminary Phase 2 data concerning safety and immunogenicity for our ACI-7104.056 active immunotherapy, currently under investigation for early-stage Parkinson’s disease. The observed immunogenicity after just three months of treatment, along with a consistently favorable safety profile, underscores the superior qualities of our clinically validated anti-a-syn active immunotherapy for managing Parkinson’s disease. We anticipate sharing more information in the first half of 2025, particularly regarding the potential progression into Part 2 of the VacSYn trial.”
Dr. Pfeifer further noted, “As a frontrunner in active immunotherapy for neurodegenerative disorders, supported by two candidates designated as FDA Fast Track, we are excited about these early VacSYn findings. They reinforce our strategy of employing active immunotherapies to target the key pathological proteins associated with neurodegenerative diseases, like a-synuclein in Parkinson’s disease, before irreversible harm occurs.”
VacSYn is an adaptive, placebo-controlled, biomarker-focused Phase 2 trial involving early PD patients and consists of two interconnected parts. The first part includes preliminary assessments from over 30 participants who were randomized to receive either ACI-7104.56 or a placebo at a 3:1 ratio. To date, the only reported safety concerns have been transient injection site reactions (49%) and headaches (18%).
The interim analysis demonstrates that significant antibody responses were effectively generated against the target antigen by week 6 following two immunizations and were found to be highly boostable. Treatment with ACI-7104.056 resulted in an average increase of anti-a-syn antibodies that was 16 times greater than the placebo level after three immunization sessions.
Depending on additional interim findings expected in the first half of 2025, including pharmacodynamic data, AC Immune may opt to commence Part 2 of VacSYn, which could involve up to 150 participants. Patients in this phase will also be monitored for the progression of both motor and non-motor symptoms of the disease, in addition to digital, imaging, and fluid biomarkers. The goal is to establish early proof-of-concept and identify disease-specific biomarkers to facilitate a swift transition into a pivotal trial.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
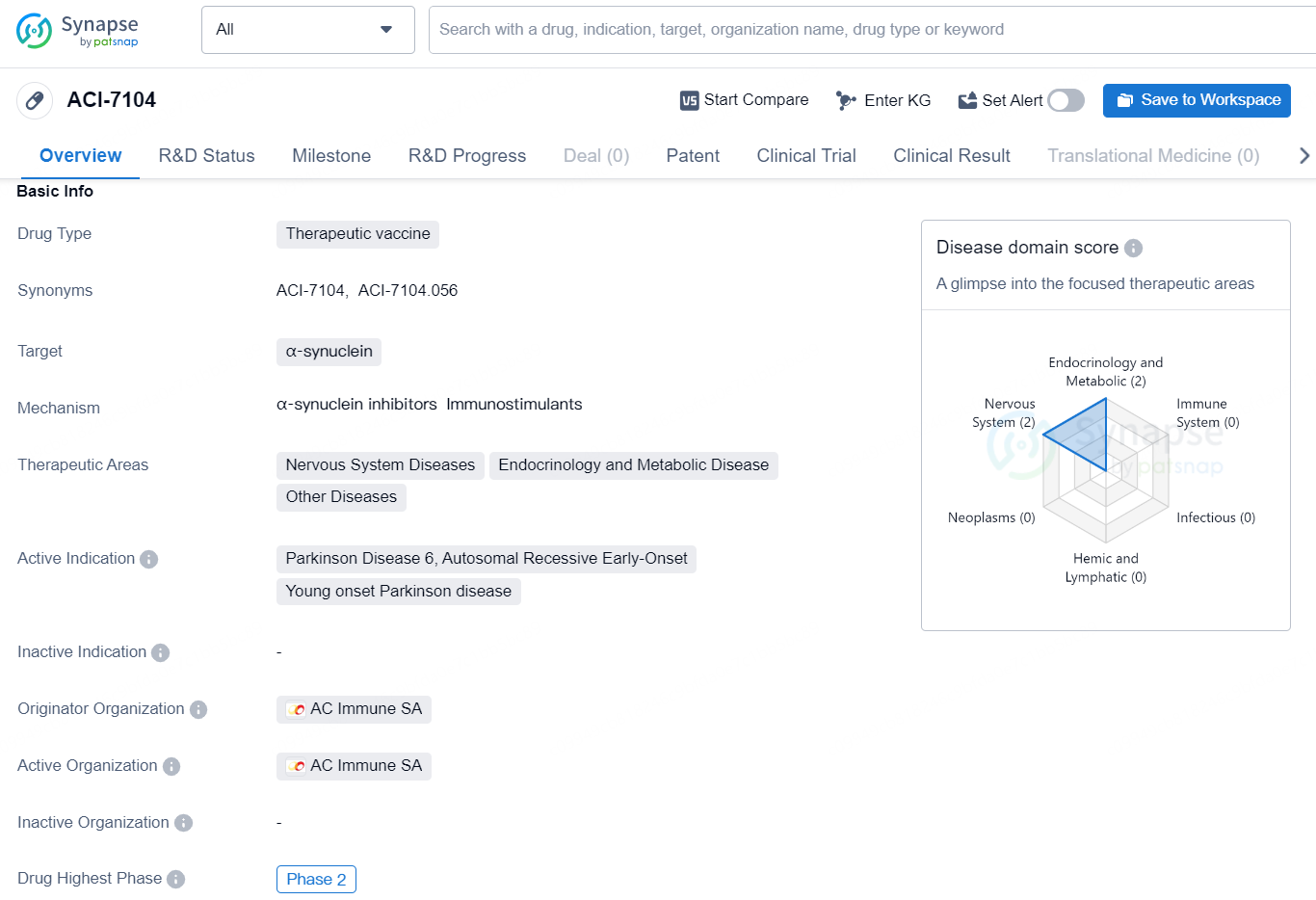
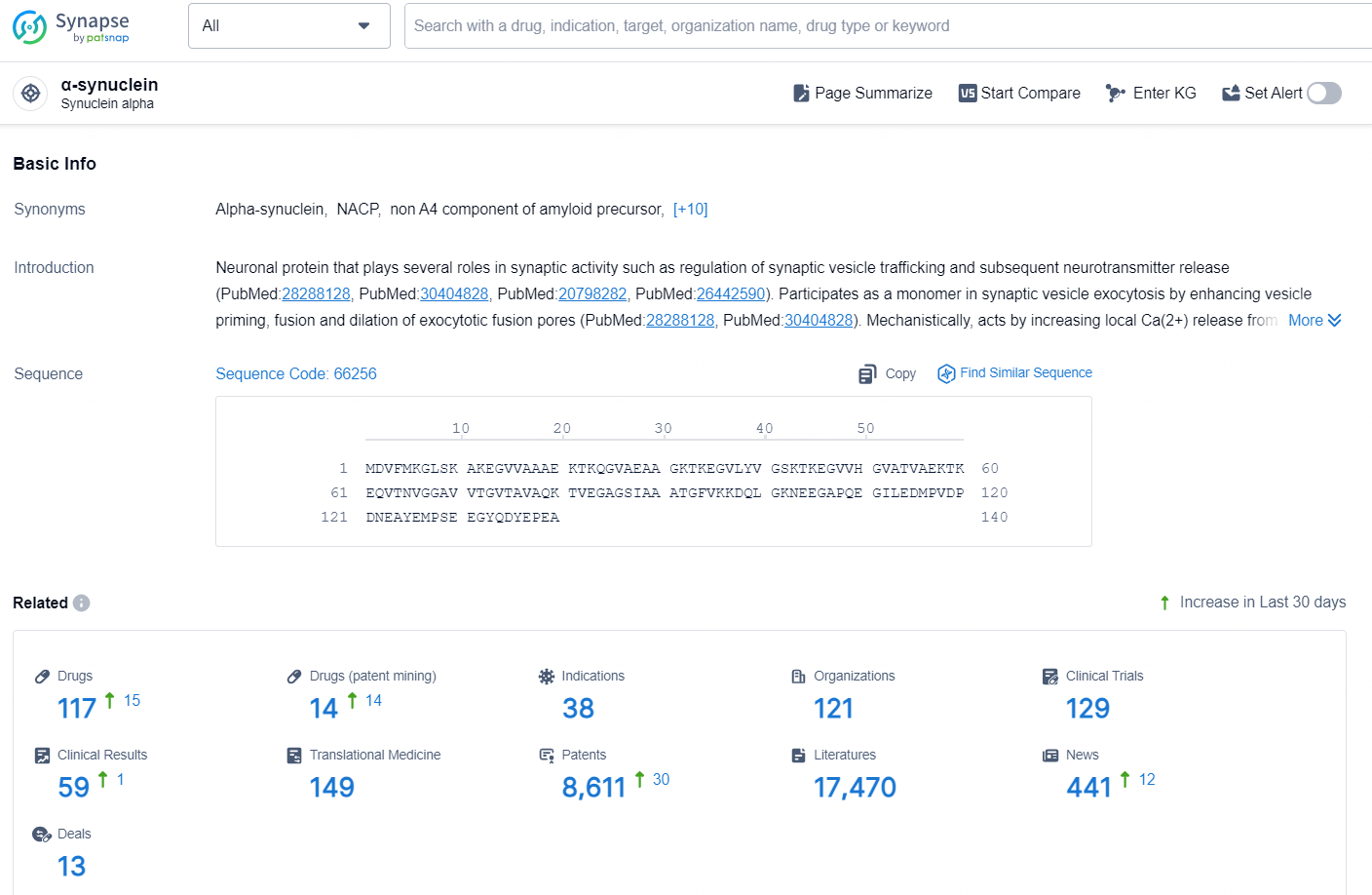
According to the data provided by the Synapse Database, As of November 19, 2024, there are 117 investigational drug for the α-synuclein target, including 38 indications, 121 R&D institutions involved, with related clinical trials reaching 129, and as many as 8611 patents.
ACI-7104 is a therapeutic vaccine that targets α-synuclein and is designed to address nervous system diseases, endocrinology and metabolic disease, and other diseases. This drug is specifically indicated for Parkinson Disease 6, Autosomal Recessive Early-Onset, and Young onset Parkinson disease. The drug is in the highest phase of development, which is Phase 2, and the originator organization is AC Immune SA.