Blenrep Shows Enhanced Survival in DREAMM-7 Trial for Relapsed/Refractory Multiple Myeloma
GSK plc (LSE/NYSE: GSK) has revealed encouraging initial findings from a scheduled interim assessment of the DREAMM-7 head-to-head phase III study. This trial examines the efficacy of Blenrep (belantamab mafodotin) in conjunction with bortezomib and dexamethasone (BorDex) as a treatment option for relapsed or refractory multiple myeloma after the first line. The trial successfully achieved its primary secondary goal of overall survival (OS), demonstrating that the combination of belantamab mafodotin and BorDex significantly lowers the mortality risk compared to the standard treatment of daratumumab plus BorDex.
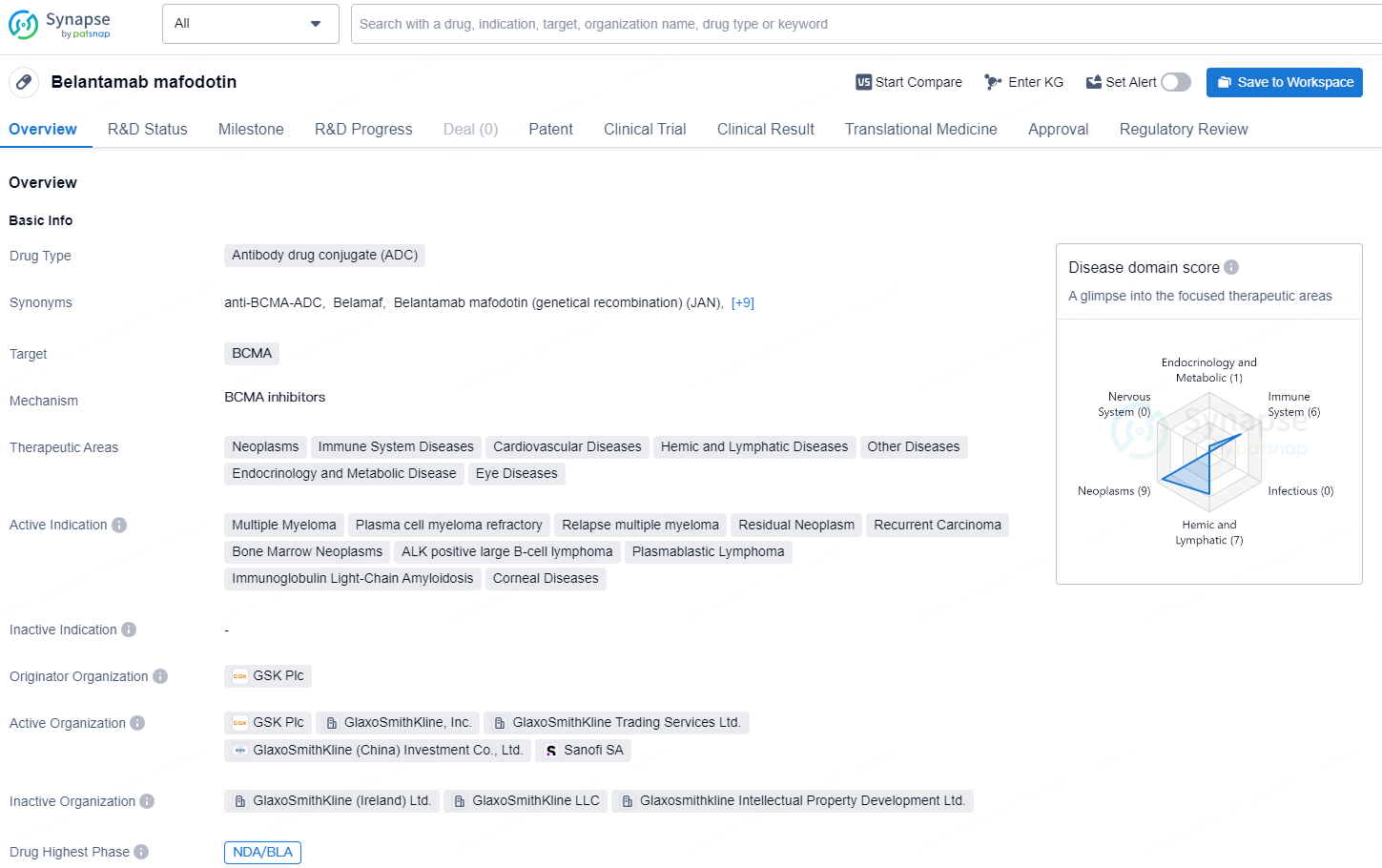
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Hesham Abdullah, Senior Vice President and Global Head of Oncology at R&D for GSK, commented: "The overall survival outcomes from the DREAMM-7 clinical trial highlight the potential of this Blenrep combination to enhance the lifespan of patients suffering from relapsed/refractory multiple myeloma. This marks a statistically significant and clinically impactful development for patients, which could be transformative for their treatment. We eagerly anticipate sharing these findings with regulatory bodies and presenting the comprehensive results at the American Society of Hematology Annual Meeting next month."
The interim analysis results, including safety information, will be showcased at the 66th Annual Meeting and Exposition of the American Society of Hematology (ASH) scheduled for December 9, 2024, at 11:15 a.m. PT.
The DREAMM (DRiving Excellence in Approaches to Multiple Myeloma) clinical program is ongoing and continues to assess the efficacy of belantamab mafodotin in early treatment stages, as well as in conjunction with innovative therapies and standard treatment options. Beyond DREAMM-7, this includes the current head-to-head phase III DREAMM-8 trial that compares belantamab mafodotin paired with pomalidomide and dexamethasone against bortezomib combined with pomalidomide and dexamethasone.
A phase III trial involving newly diagnosed multiple myeloma patients who are not eligible for transplants is expected to commence by the end of 2024 as part of the DREAMM initiative.
In 2024, submissions for belantamab mafodotin combinations have been made in the US, EU, Japan, UK, Canada, and Switzerland aimed at treating relapsed or refractory multiple myeloma, informed by data from the DREAMM-7 and DREAMM-8 studies. In China, the National Medical Products Administration has awarded Breakthrough Therapy Designation for belantamab mafodotin used in combination with BorDex and has prioritized the review of the regulatory application based on the findings from DREAMM-7.
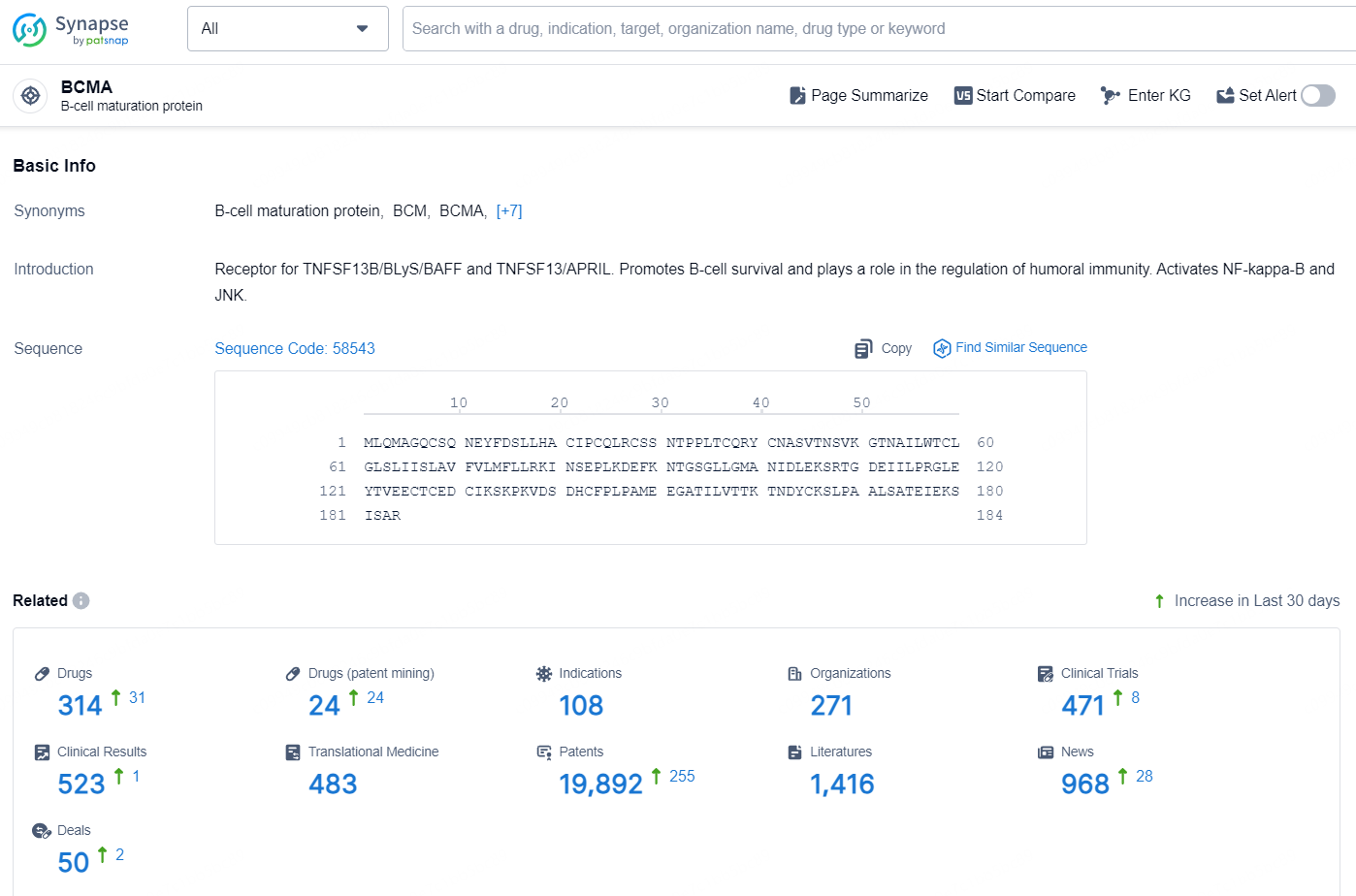
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 19, 2024, there are 314 investigational drugs for the BCMA target, including 108 indication, 271 R&D institutions involved, with related clinical trial reaching 471, and as many as 19892 patents.
Belantamab mafodotin is an antibody drug conjugate (ADC) that targets BCMA and is indicated for neoplasms, immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, other diseases, endocrinology and metabolic disease, and eye diseases.