Exploring Risperidone: Its Impact, Mechanism, and Innovations in Psychiatric Treatment
Risperidone is a small molecule drug that acts on the 5-HT2A and D2 receptors. It is used in the treatment of various conditions, including bipolar I disorder, irritable mood, autistic disorder, bipolar disorder, schizophrenia, and schizoaffective disorder. The drug was first approved in the United States in December 1993 and is indicated for various other diseases. The originator organization for Risperidone is Johnson & Johnson, a multinational pharmaceutical and consumer goods company. The drug has achieved the highest phase of approval in both the global market and China, indicating its widespread acceptance and adoption in the treatment of the aforementioned conditions.
Risperidone is widely used in the management of psychiatric and neurological disorders and has been a significant contributor to the treatment of these conditions since its approval. It has demonstrated efficacy in addressing the symptoms associated with bipolar disorder, schizophrenia, and related mood disorders, making it a valuable option for healthcare professionals and patients.
Below, we will use the drug Risperidone as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
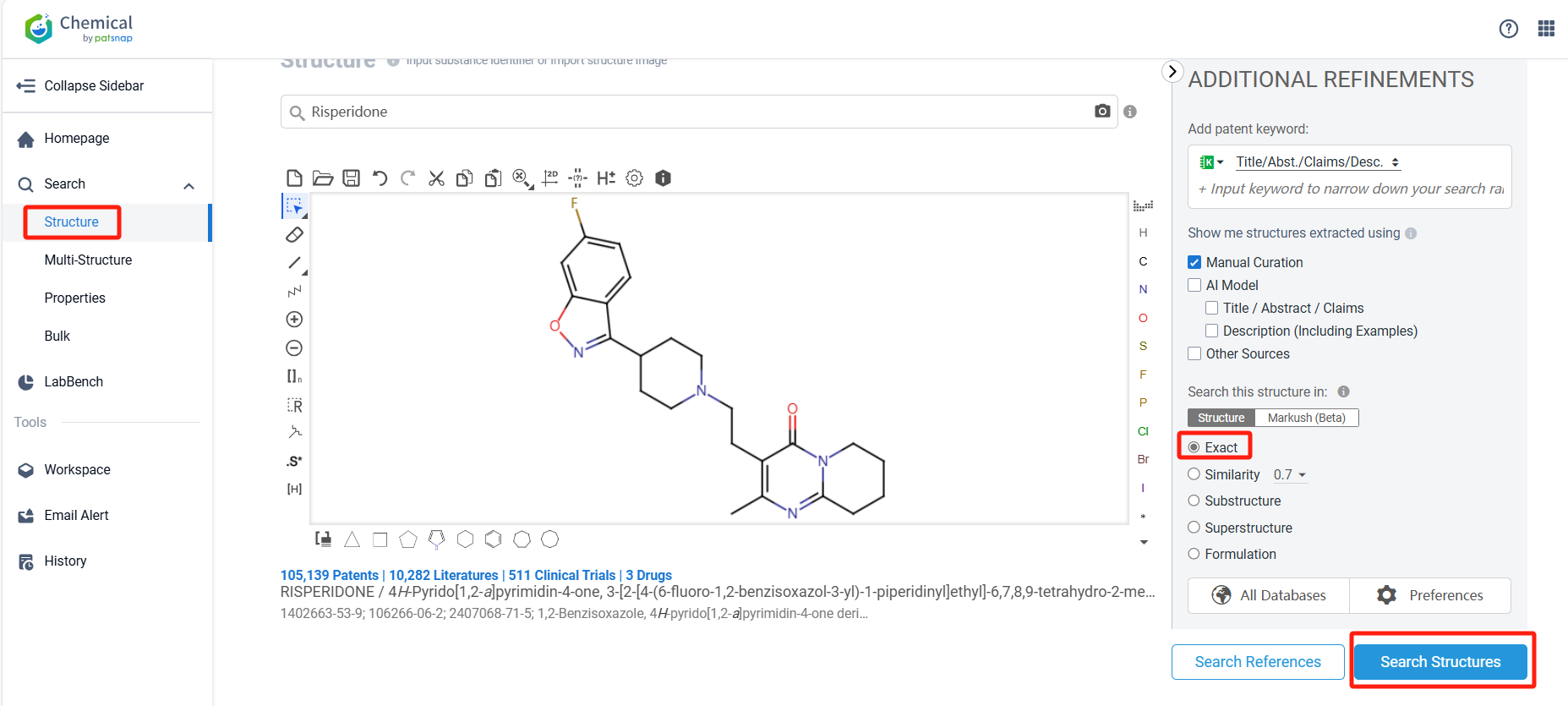
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of Risperidone (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, we select "Exact Search", click on search structures, and you can find 2034 patents. In the sidebar, select "Formulation" and "Use" under the "Claim Types" to search for patents related to new formulations and new indications. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
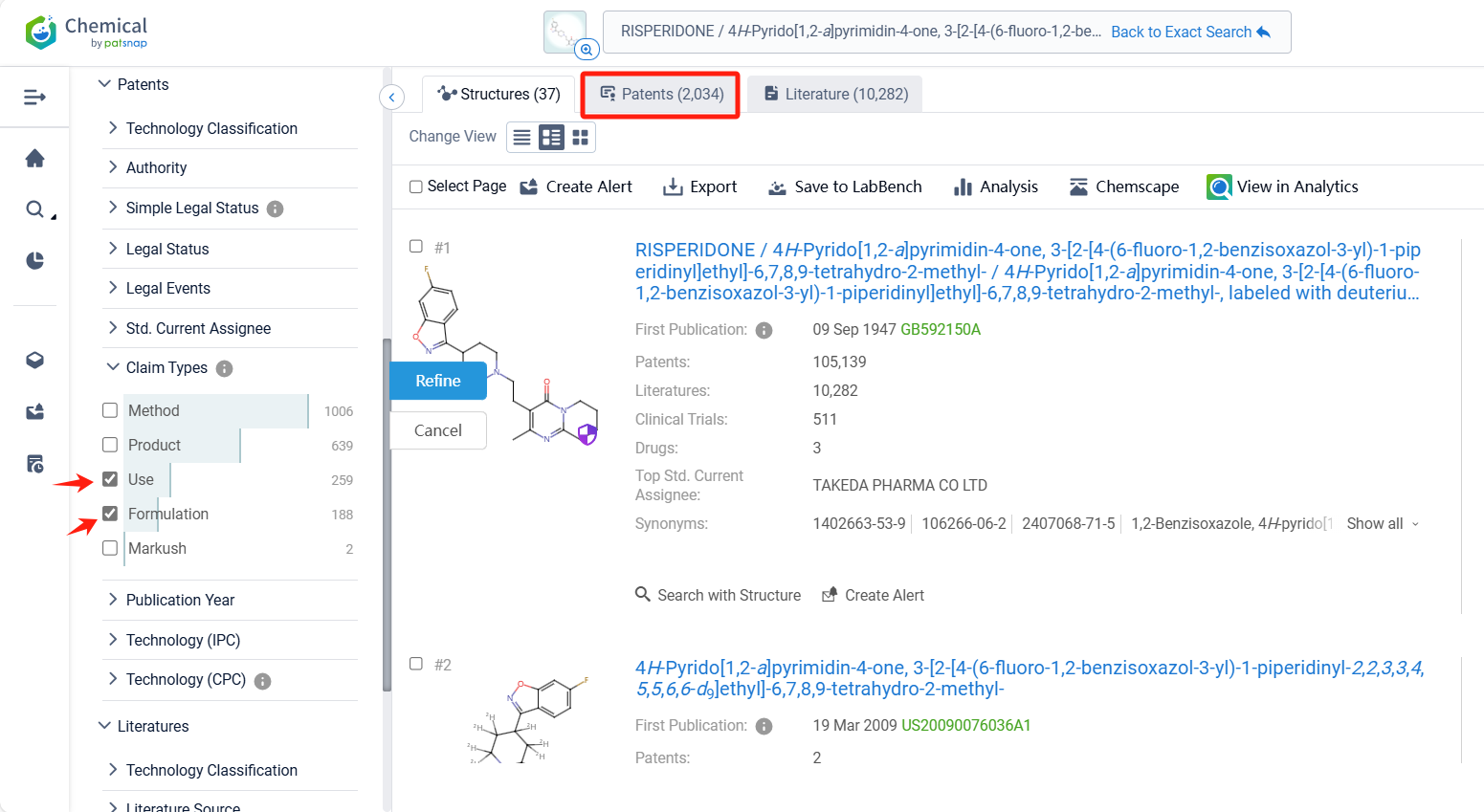
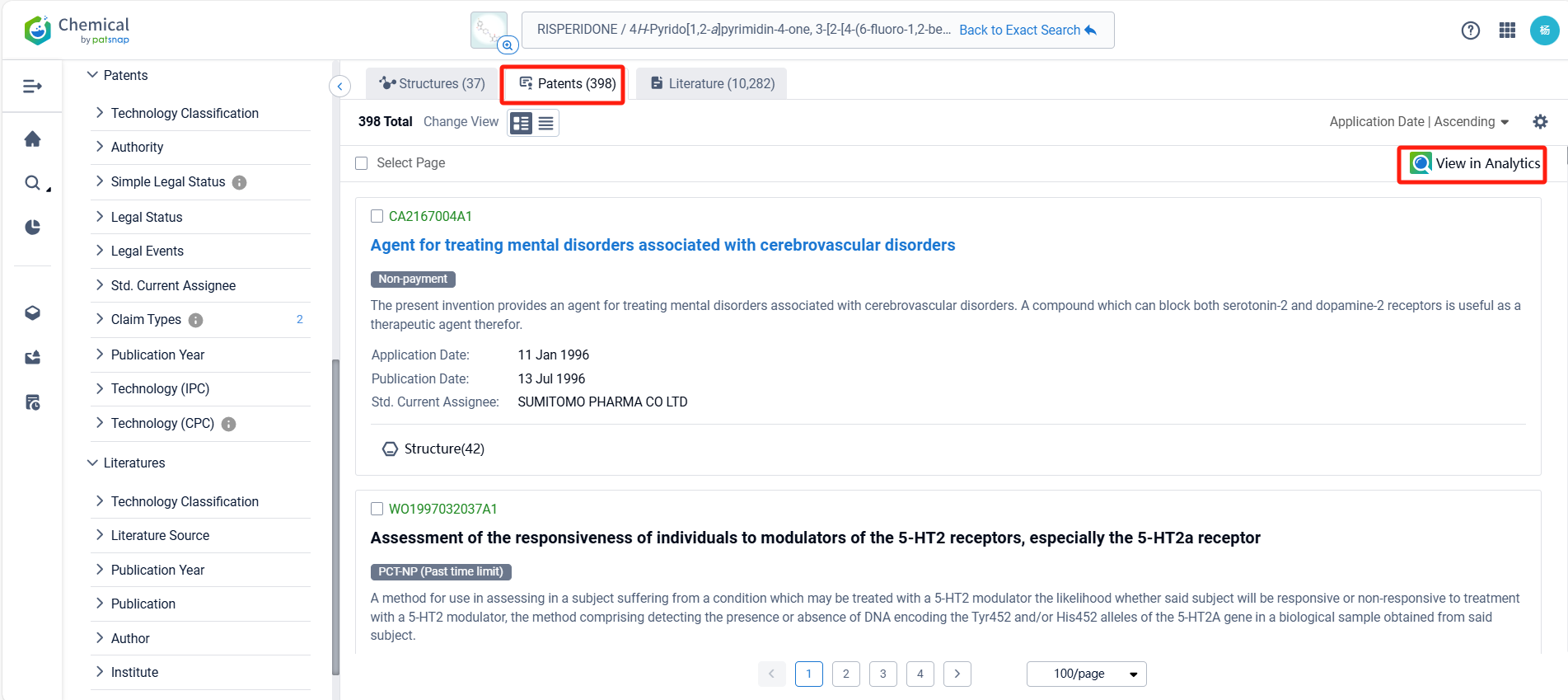
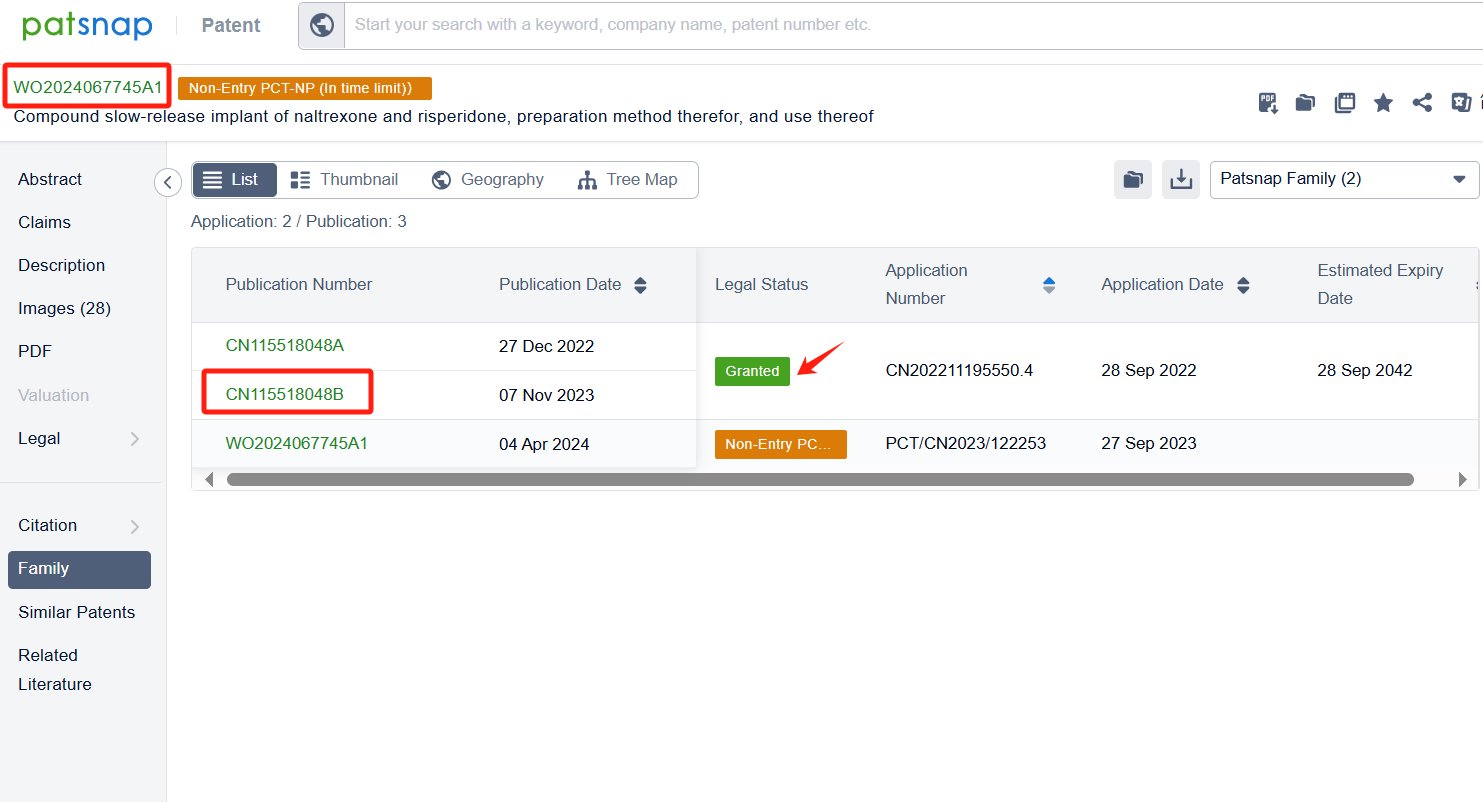
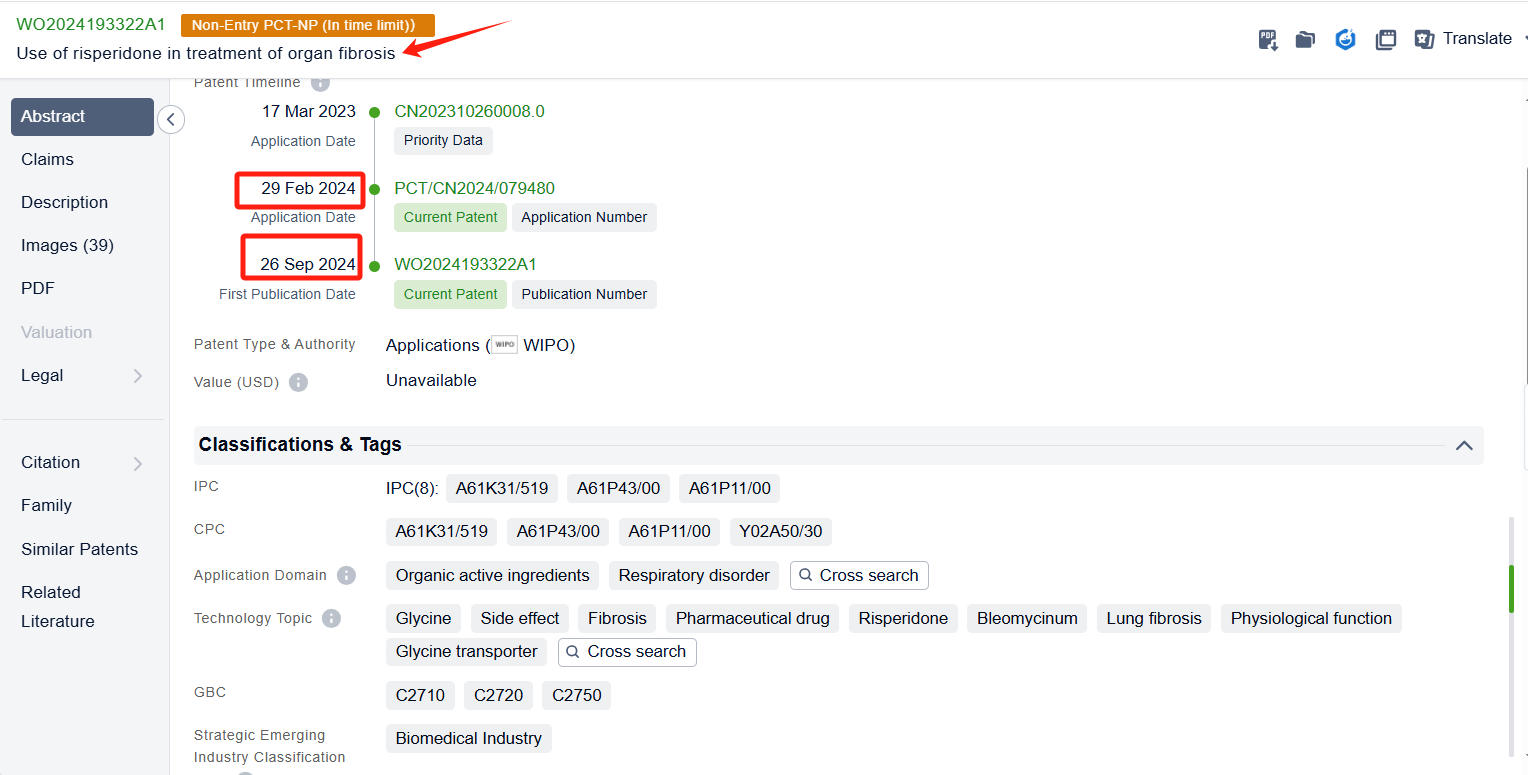
In the Patsnap patent database, we can sort patents by their publication dates to identify the latest patents on Risperidone. By reviewing the aforementioned patents, we can observe that Shenzhen Sciencare Pharmaceutical Co., Ltd.'s international patent WO2024067745A1 (application date 20230927, publication date 20240404) discloses a compound sustained-release implant of naltrexone and risperidone, The implant has an appropriate drug delivery system and can maintain stable blood drug concentrations in the body for a long time. Its Chinese counterpart patent CN115518048B has been granted on November 7, 2023.Additionally, Tianjin Medical University's international patent WO2024193322A1 (application date 20240229, publication date 20240926) relates to the application of risperidone in treating organ fibrosis, by inhibiting the glycine transporter GLYT1 and inhibiting collagen synthesis, risperidone can significantly improve the pulmonary fibrosis caused by bleomycin, and also significantly improve the already formed pulmonary fibrosis.
As a small molecule drug, Risperidone's mechanism of action involves targeting the 5-HT2A and D2 receptors, which are implicated in the pathophysiology of various psychiatric and neurological conditions. Its approval in multiple countries, including the United States and China, highlights its global significance and accessibility to patients in need of such treatment options.
Overall, Risperidone's approval and widespread use in treating various psychiatric and neurological disorders underscore its importance in the pharmaceutical industry and its impact on patient care. The drug has been a valuable addition to the treatment armamentarium for healthcare professionals, providing options for managing the symptoms and improving the quality of life for patients affected by these conditions.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.