Unlocking the Therapeutic Potential of Imetelstat: Chemical Modifications in ASO Drug Design
Antisense oligonucleotide (ASO) drugs represent a significant branch of the small nucleic acid therapeutic field, functioning as molecules that regulate gene expression through specific binding to mRNA. Typically designed with 15–25 nucleotides, they interact with target mRNA via Watson-Crick base pairing, thereby suppressing specific gene activity. The mechanisms of action of ASO drugs primarily include two pathways: first, activation of endogenous RNase H1 enzymes to degrade the target mRNA; second, a steric hindrance mechanism that blocks critical regions of mRNA, impacting its maturation or translation into proteins. By specifically binding to target mRNA, ASOs modulate gene expression to treat related diseases. The global ASO drug market is rapidly growing, with projected sales exceeding $10 billion by 2025.
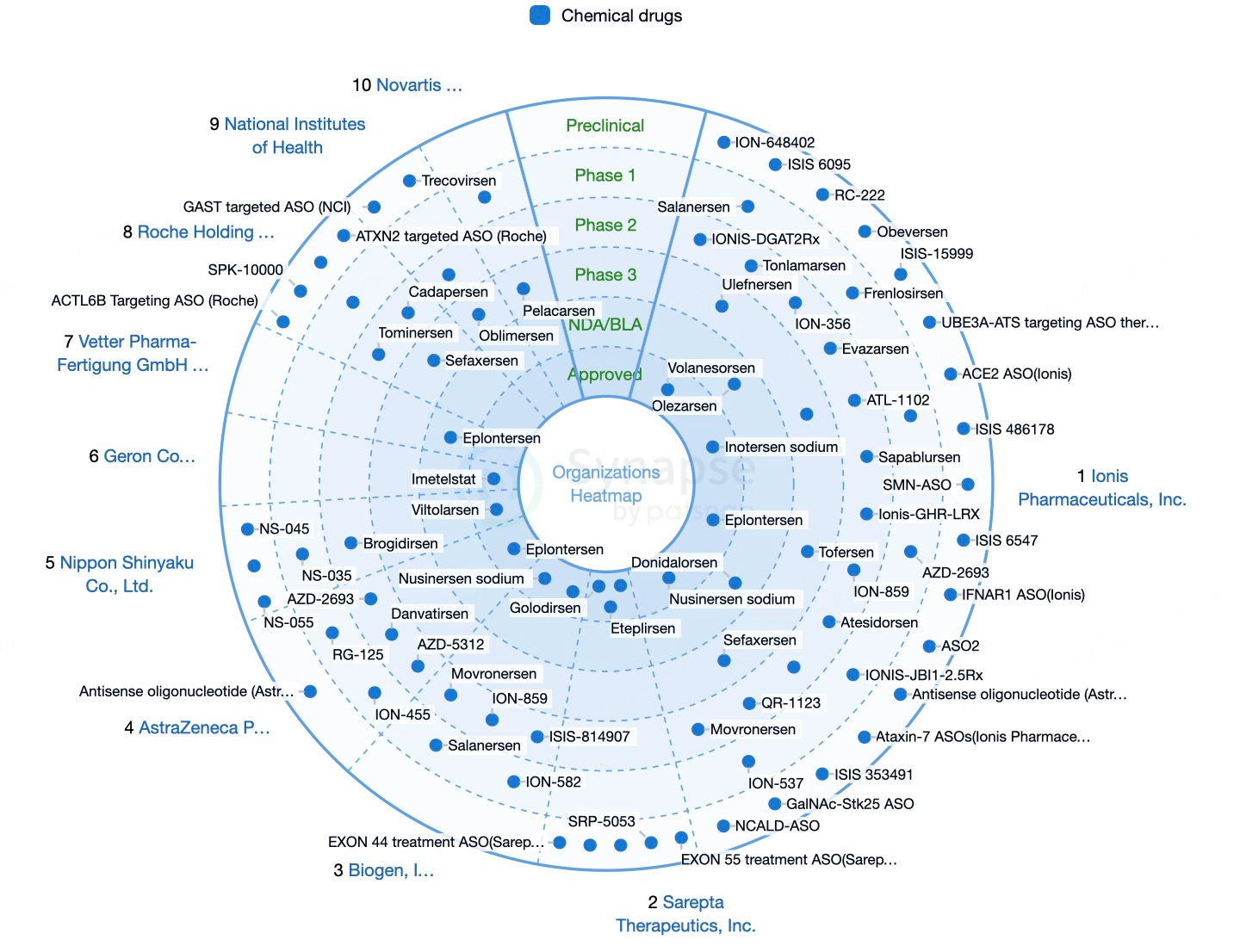
Since the first ASO drug, Fomivirsen, was approved in 1999, 13 ASO drugs have successfully entered the global market. Among these, Ionis Pharmaceuticals has developed six ASO drugs, establishing itself as a leader in this field. ASO drugs have shown great promise in treating genetic diseases, with notable progress in addressing Duchenne muscular dystrophy, lipoprotein lipase deficiency, and spinal muscular atrophy.
In June 2024, Geron Corporation's ASO drug Imetelstat (brand name: RYTELO) received FDA approval for treating adult patients with low- to intermediate-1 risk myelodysplastic syndromes (MDS) who are ineligible for erythropoiesis-stimulating agents (ESAs) or are unresponsive or resistant to them.
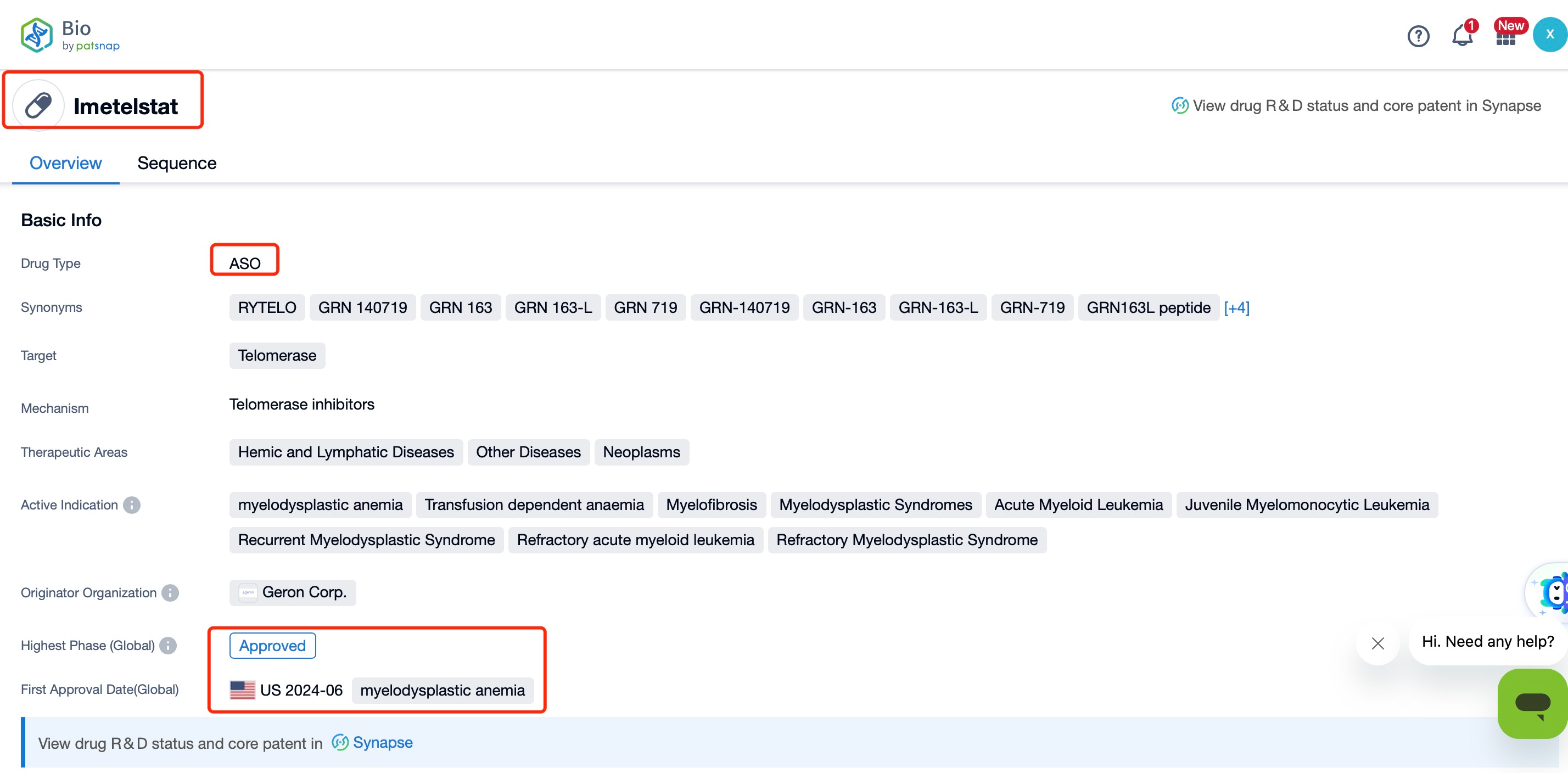
Imetelstat acts by specifically binding to and inhibiting telomerase activity, thereby disrupting its normal function in cancer cells. Telomerase is hyperactive in many cancer cells, enabling their uncontrolled proliferation. By inhibiting telomerase, Imetelstat induces telomere shortening, ultimately affecting cancer cell division and survival. This mechanism distinguishes Imetelstat from conventional ASO drugs, which typically achieve therapeutic effects by hybridizing with target RNA to interfere with RNA expression and function. In contrast, Imetelstat regulates the apoptosis of malignant hematopoietic stem cells by inhibiting telomerase activity, reducing the proliferation of malignant stem and progenitor cells, and treating MDS and related diseases.
To further explore the mechanism of action of Imetelstat, we used Patsnap Bio to investigate its sequence and clinical studies. This database provides insights into the biological mechanisms of Imetelstat and its development progress worldwide.
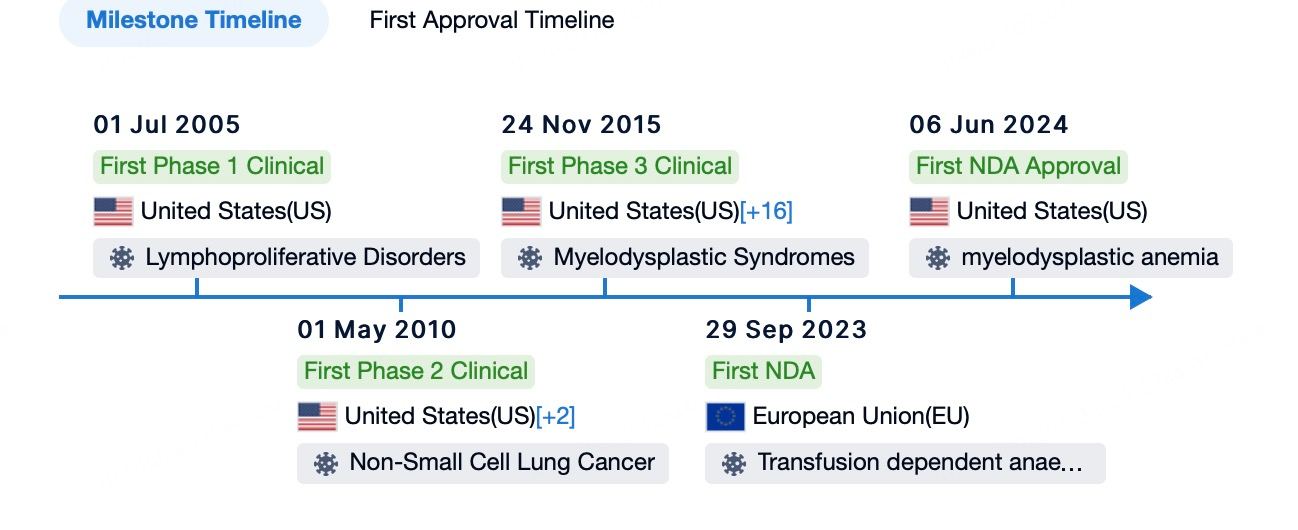
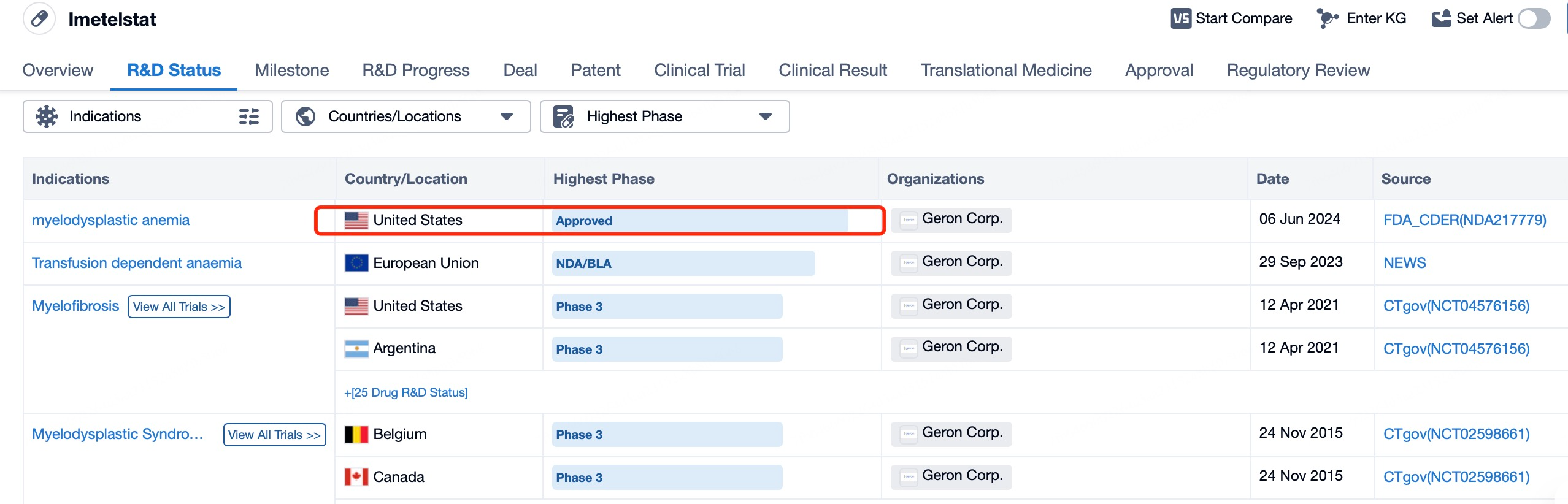
Using Patsnap Bio and Patsnap Synapse, we found that the Phase 3 IMerge clinical trial of Imetelstat, conducted between September 11, 2019, and October 13, 2021, enrolled 178 patients who were randomly assigned to receive Imetelstat (118 patients) or placebo (60 patients). The trial results showed that 40% of patients in the Imetelstat group achieved at least 8 weeks of red blood cell transfusion independence (RBC-TI), compared to 15% in the placebo group, indicating significantly better efficacy for Imetelstat (p = 0.0008). The median follow-up time was 19.5 months in the Imetelstat group and 17.5 months in the placebo group. Regarding safety, 91% of patients in the Imetelstat group experienced grade 3–4 adverse events, compared to 47% in the placebo group. The most common grade 3–4 adverse events in the Imetelstat group were neutropenia (68%) and thrombocytopenia (62%), compared to 3% and 8%, respectively, in the placebo group. No treatment-related deaths were reported. These data supported the FDA approval of Imetelstat for adult patients with low- to intermediate-1 risk MDS who are ineligible for or unresponsive to ESAs. This approval was based on the results of the Phase 3 IMerge trial conducted across 17 countries at 118 research centers, including university hospitals, cancer centers, and outpatient clinics.

Structurally, Imetelstat is a 13-nucleotide (nt) ASO that specifically binds to the telomerase RNA component (TERC), competitively inhibiting telomerase activity. By inhibiting telomerase, it induces telomere shortening, thereby affecting the division and survival of cancer cells. Additionally, a search in Patsnap Bio reveals that Imetelstat can also regulate molecular pathways involved in fatty acid metabolism (e.g., FADS2 and ACSL4), inducing cell death through the formation of excessive lipid reactive oxygen species (ROS) and ferroptosis. This indicates that Imetelstat’s mechanism of action may extend beyond telomerase inhibition, involving impacts on cellular metabolism.
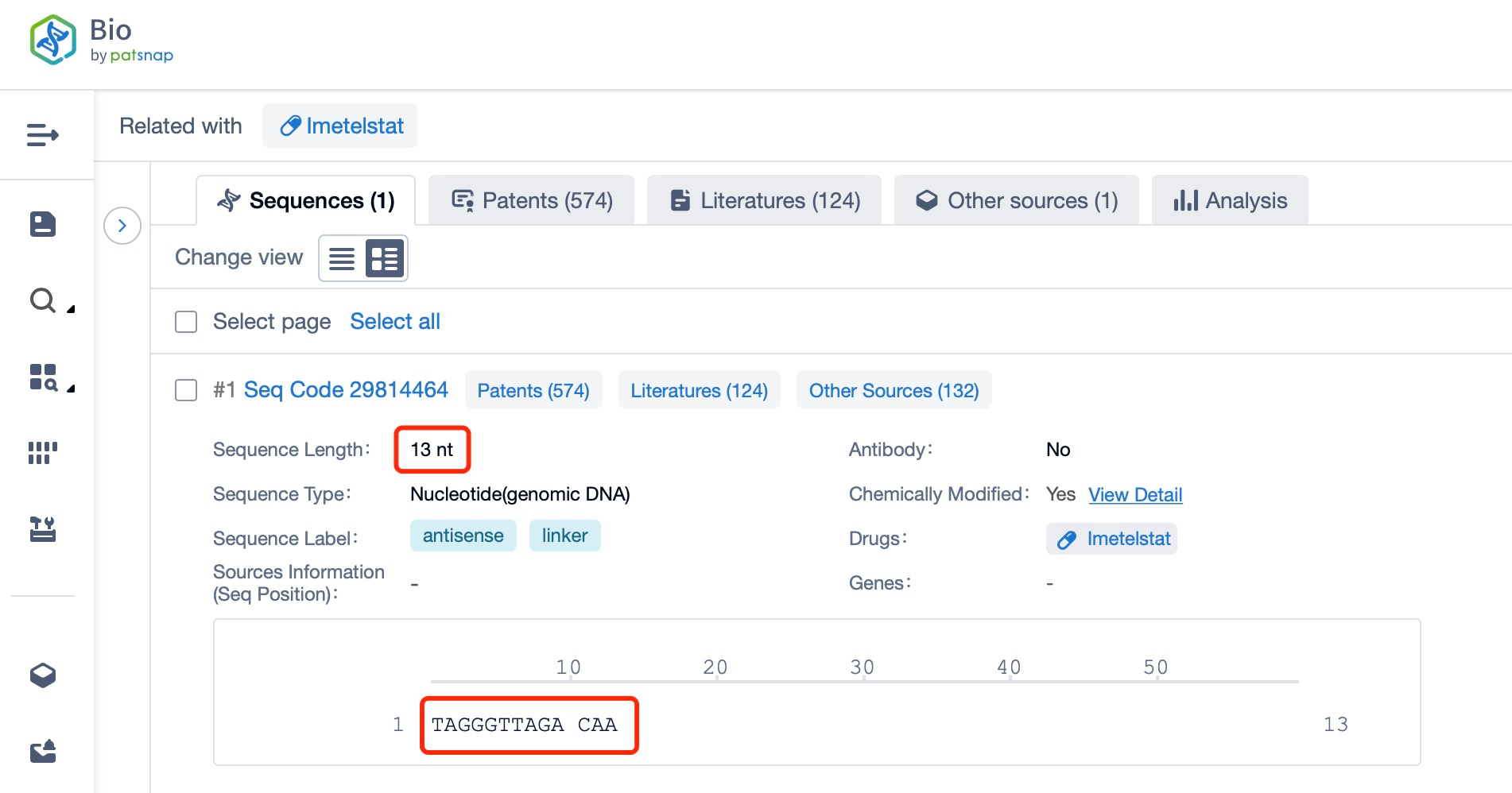
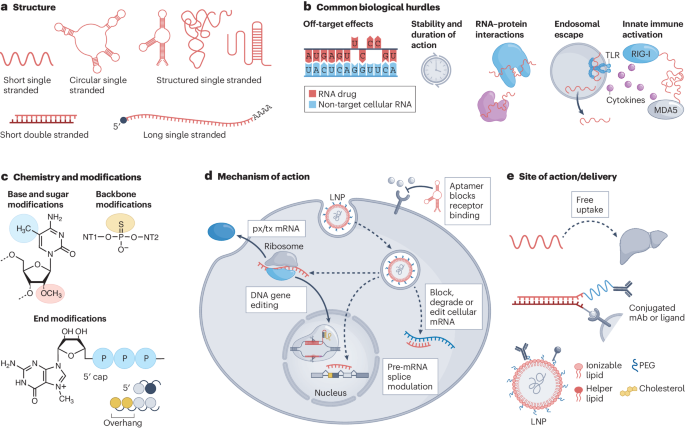
Chemical modifications are crucial for the efficacy and stability of ASO drugs. According to information in Patsnap Bio, Imetelstat's chemical structure incorporates several modifications, including 2'-O-methyl (2'-OMe), sugar-phosphate backbone modifications, 3'-end modifications, and lipid conjugation.
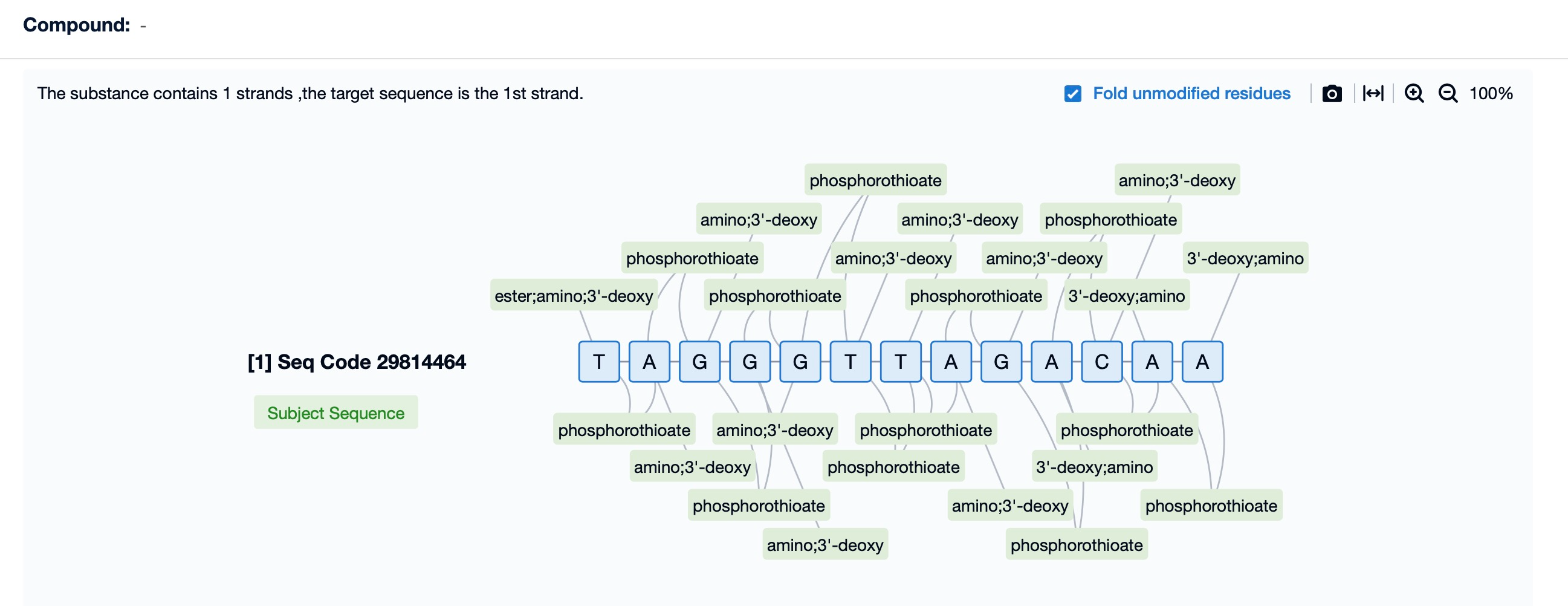
Among these, the 2'-OMe modification is a key strategy in ASO drug design, enhancing drug stability and efficacy. By introducing a methyl group at the 2' position of nucleotides, the molecule's metabolic stability is increased, reducing degradation by endogenous nucleases and improving its half-life and bioavailability in vivo. This modification also enhances the binding affinity of Imetelstat to its target mRNA, thereby increasing specificity and potency. Furthermore, 2'-OMe modifications improve pharmacokinetic properties, reduce dosing frequency, and minimize immune responses and toxicity, which are critical for treating certain genetic disorders.
In Imetelstat’s sugar-phosphate backbone, the replacement of non-bridging oxygen atoms with sulfur, known as phosphorothioate modification, is another common ASO modification. This modification further enhances ASO stability by resisting nuclease degradation while positively influencing pharmacokinetic properties. Phosphorothioate modifications increase resistance to nucleases in serum and tissues, prolonging the drug’s half-life and potentially improving its pharmacokinetics. This backbone modification is a key strategy for optimizing Imetelstat's performance as a telomerase inhibitor.
Additionally, the introduction of amino modifications at the 3'-end of Imetelstat further enhances its binding affinity to target mRNA, increasing the specificity and potency of the ASO. This strategy enables Imetelstat to more effectively inhibit its target RNA, delivering remarkable therapeutic effects for related diseases.
Lipid conjugation enhances Imetelstat’s intracellular delivery capability, enabling it to enter cells effectively and perform its functions. This modification improves resistance to serum and tissue nucleases, extending the drug’s circulation half-life. Lipid-conjugated molecules interact more effectively with cell membranes, facilitating cellular uptake through direct penetration or endocytosis and increasing bioavailability. This modification also improves the drug's distribution in vivo, allowing for targeted accumulation in specific tissues. Additionally, lipid conjugation helps reduce immune responses and potential toxicity, ensuring a balance of efficacy and safety.
Summary
Imetelstat is a 13-nucleotide ASO that inhibits telomerase activity by specifically binding to TERC, thereby blocking its normal function in cancer cells. Telomerase is hyperactive in many cancer cells, facilitating their unlimited proliferation. Imetelstat inhibits telomerase, leading to telomere shortening and impacting cancer cell division and survival. This mechanism sets Imetelstat apart from traditional ASO drugs, which typically target RNA through specific hybridization to disrupt its expression and function. Instead, Imetelstat regulates the apoptosis of malignant hematopoietic stem cells by inhibiting telomerase activity, reducing the proliferation of malignant stem and progenitor cells, and treating myelodysplastic syndromes (MDS) and related diseases.
The chemical modifications of Imetelstat are key to its ability to inhibit telomerase activity effectively. These modifications—2'-OMe, phosphorothioate, 3'-amino, and lipid conjugation—enhance the drug’s efficacy and safety. Through these modifications, Imetelstat more effectively inhibits telomerase activity, reduces malignant cell proliferation, and plays a significant role in treating diseases like MDS. The global ASO drug market is rapidly growing, with projected sales exceeding $10 billion by 2025. With advancements in technology and market expansion, ASO drugs are poised to become the third major drug class, following small-molecule drugs and antibody therapies, offering patients more treatment options.
Better answers for better bio-innovations!
Validate novelty, eliminate risk, and innovate with confidence using the world’s largest sequence database curated from millions of patent and non-patent sources.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.
With best-in-class coverage of protein and nucleic acid sequences combined with state-of- the-art search algorithms, you’ll spend less time searching and more time bringing your bio-innovations to market.
Reference
1.Shi, Y. et al. A review of existing strategies for designing long-acting parenteral formulations: Focus on underlying mechanisms, and future perspectives. Acta Pharm Sin B 11, 2396-2415 (2021). https://doi.org:10.1016/j.apsb.2021.05.002
2.Myskova, A., Sykora, D., Kunes, J. & Maletinska, L. Lipidization as a tool toward peptide therapeutics. Drug Deliv 30, 2284685 (2023). https://doi.org:10.1080/10717544.2023.2284685