Accelerating mRNA Therapeutics Research: Harnessing Patsnap Bio for Efficient Nucleotide Sequence Retrieval and Patent Analysis
On August 24th, Moderna announced that the European Commission (EC) has approved its respiratory syncytial virus (RSV) mRNA vaccine, mRESVIA (mRNA-1345), for market authorization. This marks the world's first mRNA vaccine targeting a virus other than COVID-19 and provides protection against RSV-induced lower respiratory tract disease (RSV-LRTD) and acute respiratory disease in adults aged 60 and older. Moderna has also submitted marketing authorization applications for mRNA-1345 to several regulatory bodies worldwide. This achievement not only demonstrates the broad application potential of mRNA technology beyond COVID-19 vaccines but also signifies a breakthrough in the use of mRNA therapeutics for the prevention and treatment of other diseases.

mRNA therapeutics are leading a medical revolution by ingeniously designing messenger RNA molecules that enable human cells to produce specific proteins essential for treating or preventing certain diseases. Compared to traditional drugs, mRNA therapeutics offer unique advantages: they do not integrate into the human genome, have a transient effect, and are eventually metabolized naturally by the body, earning them the reputation of "activating the body’s internal pharmaceutical factory." This innovative approach is becoming the new favorite in drug development.
The successful development of mRNA vaccines relies heavily on the precise acquisition of nucleotide sequences and an in-depth understanding of patent information. Traditional nucleotide sequence search tools and websites include several well-established and widely used databases, such as the GenBank from the National Center for Biotechnology Information (NCBI), the EMBL-Bank from the European Molecular Biology Laboratory (EMBL), and the DNA Data Bank of Japan (DDBJ). These databases are valued for their vast data, rich sequence information, and long-standing scientific significance. They provide basic search and retrieval functions, allowing researchers to access extensive public sequence data.
Patsnap Bio offers a more seamless solution, allowing users to quickly search sequence information, perform multidimensional data exploration, and utilize its intellectual property management features. In most cases, multiple information retrieval tasks can be accomplished through a single database.
Since the sequence information for mRNA-1345 is currently confidential, we will proceed with a specific sequence example to explore how nucleotide information can be efficiently retrieved using Patsnap Bio.
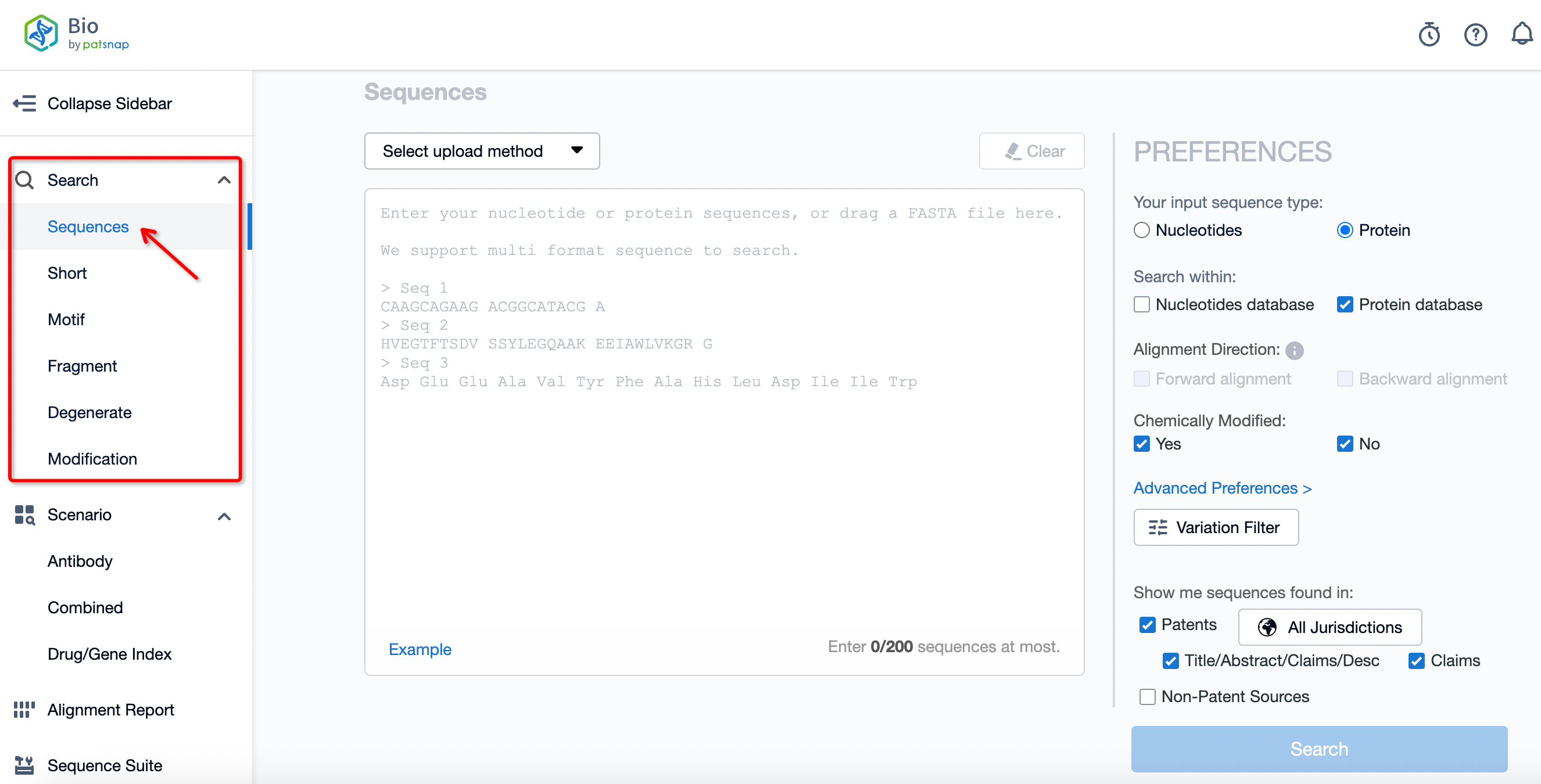
First, log in to the database's main interface through the website. Once on the homepage, you can choose from various search options based on your needs, including general search, short sequence search, motif search, fragment search, general formula search, and modification search. If you're unsure which category your search content falls under, the general search option is usually applicable.
Sequence Information Upload
Firstly, you can upload the sequence you want to retrieve as required. The method of uploading can be either by directly entering the amino acid sequence into the input box, or by selecting from the top-left corner the upload method via sequence submission or via GeneBank ID.
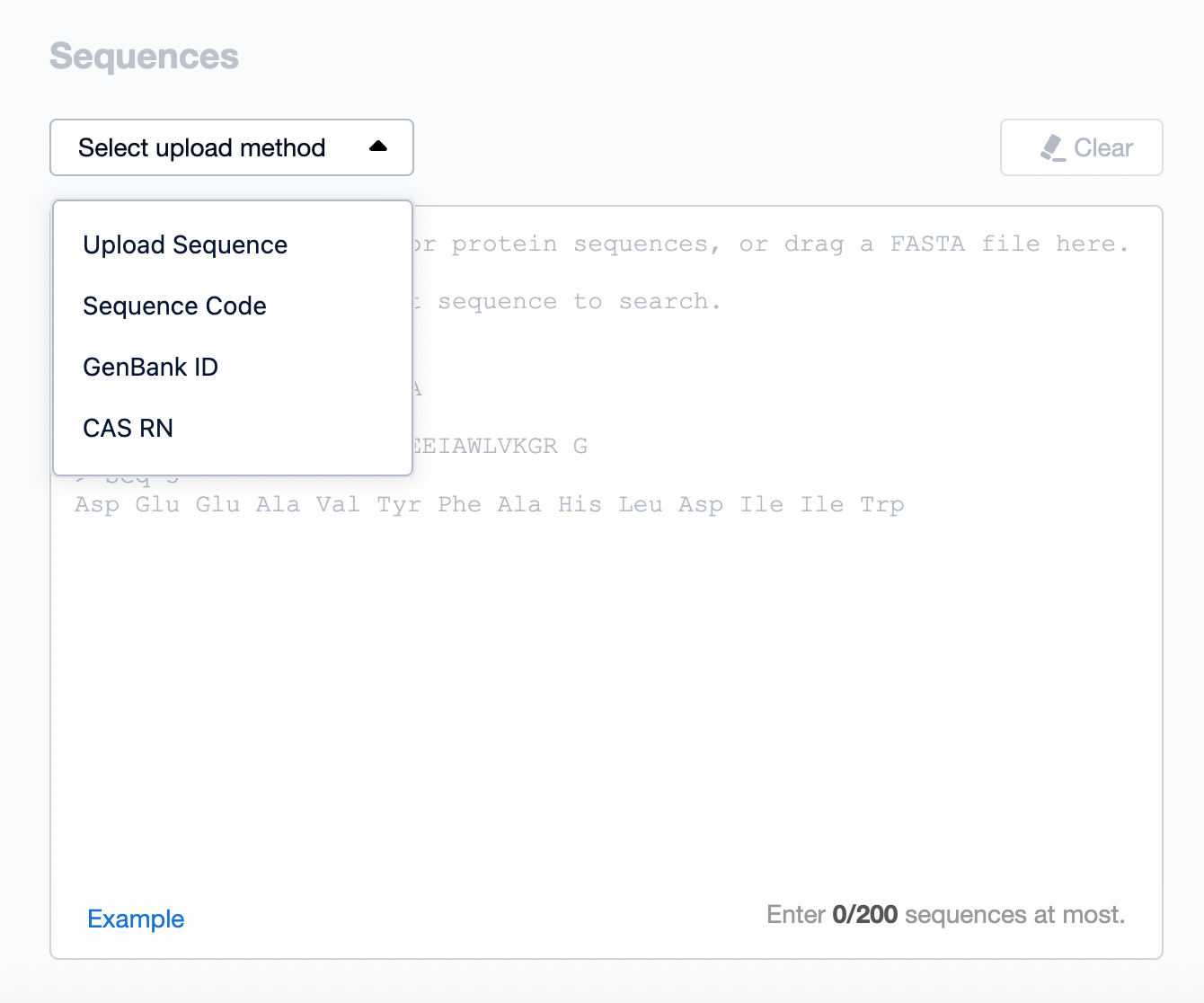
Data Upload
After uploading data, it is necessary to select the content you wish to retrieve based on your needs. If the uploaded data is a nucleotide sequence, the type should be selected as nucleotide. If the searched fragment has the capability to encode proteins, the database selection can include both nucleotide and protein databases. Generally, nucleotide sequences have more associated patents, so it is advisable to also conduct a patent search simultaneously.
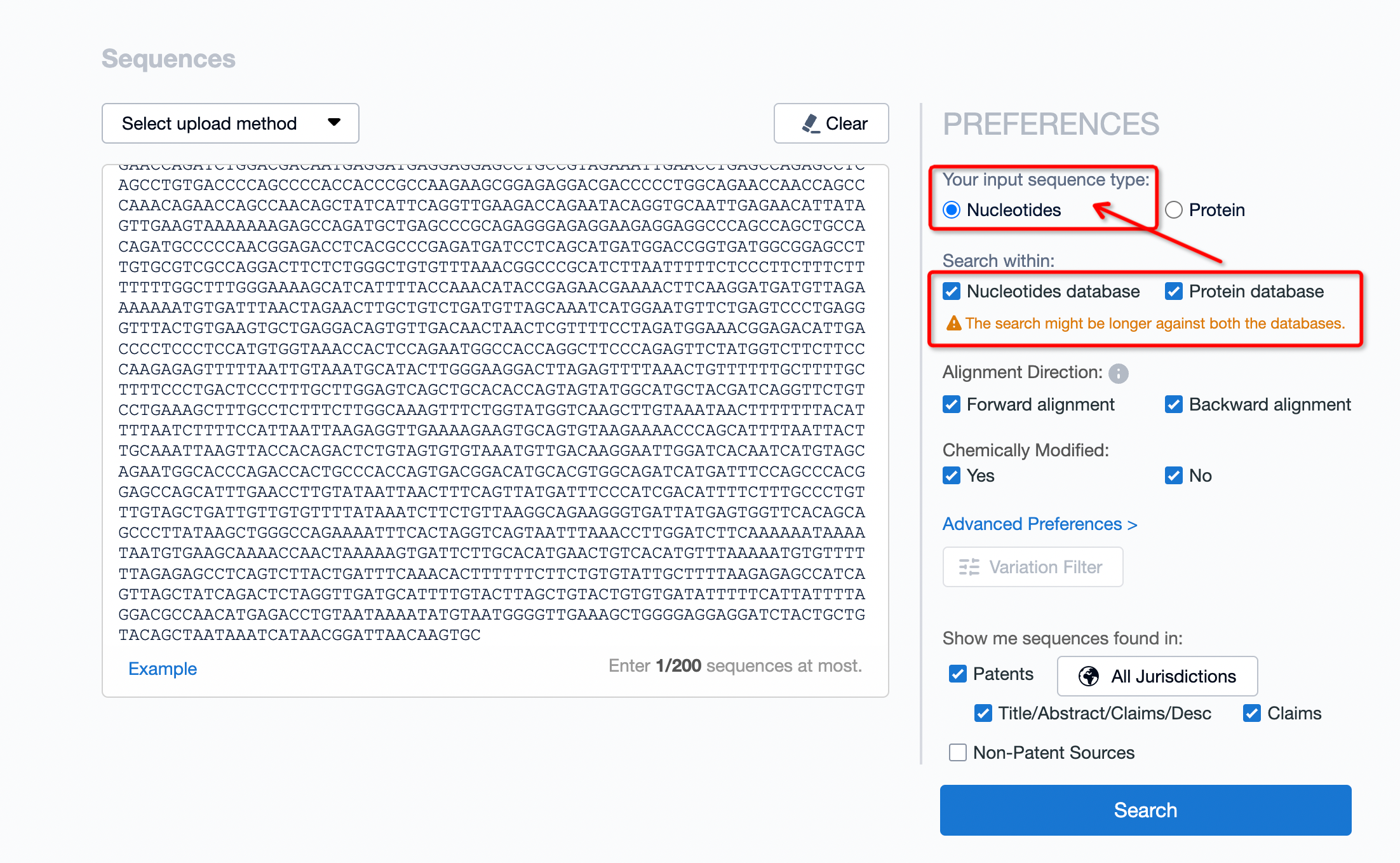
Result Analysis
Certainly, choosing to search both nucleotide and protein databases at the same time requires more time compared to querying a single database, with searches typically completed in about 5 minutes.
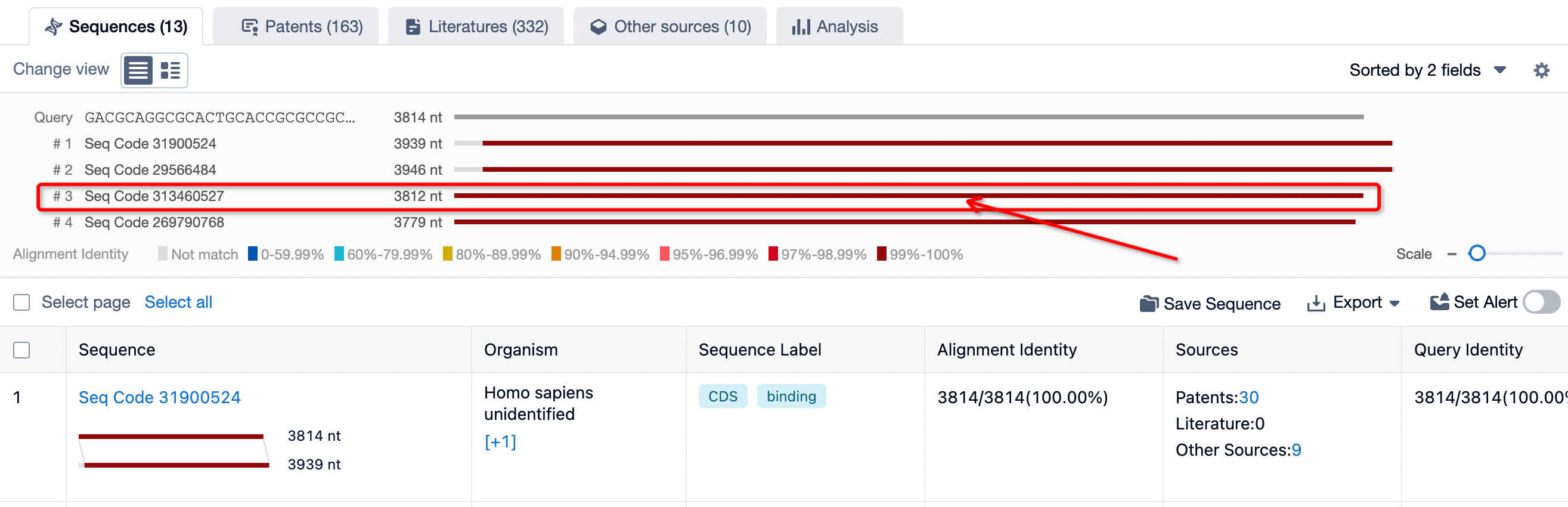
The test results are generally divided into several parts: sequences, patents, literature, other databases, and analytical views. Taking sequences as an example, let's look at the specific information detected. If there are specific requirements for sequence information, the search criteria can be restricted based on the needs to precisely locate the sequence to be queried.
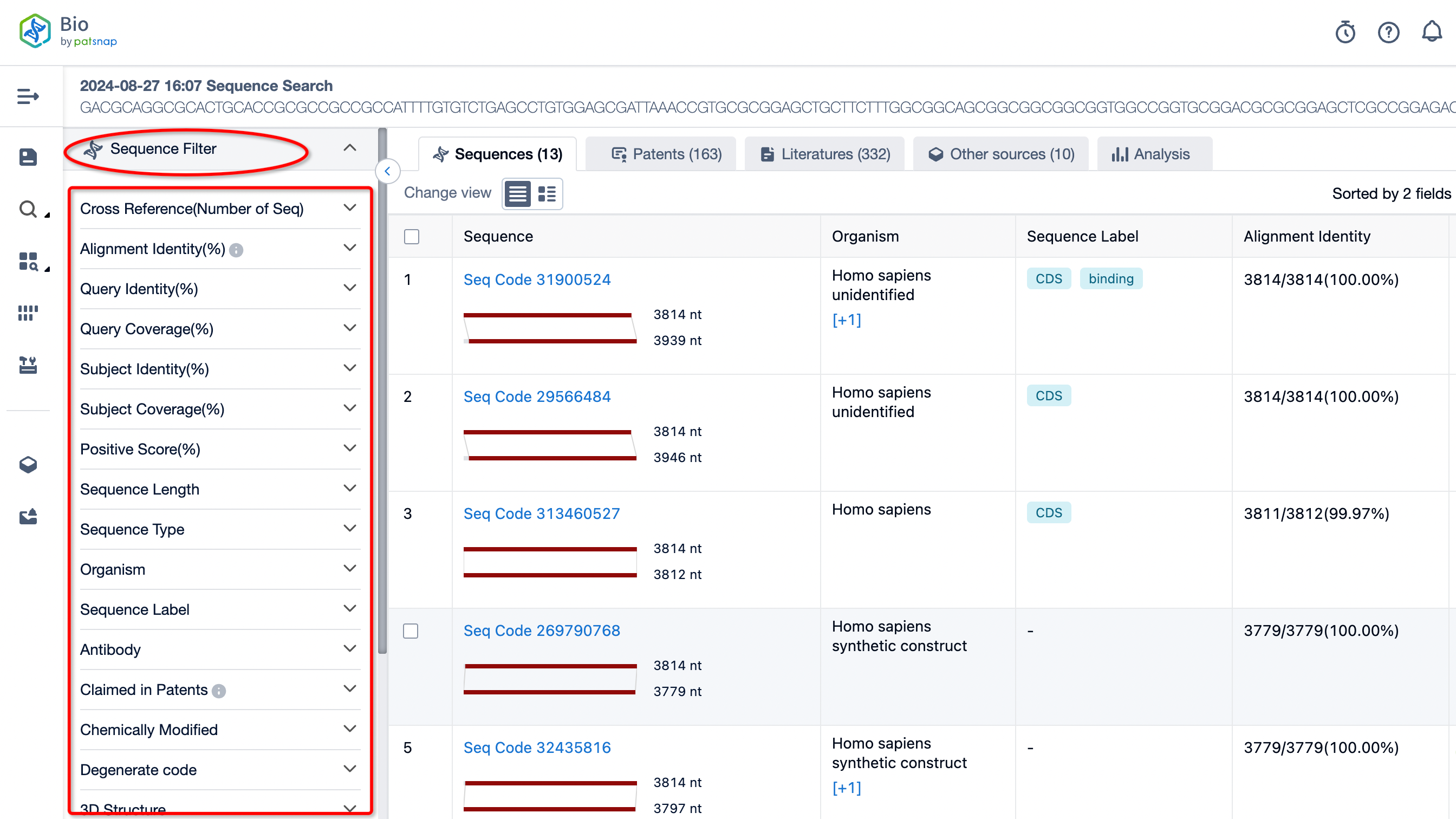
After defining the precise requirements of the sequence, one can query only the sections that match the target sequence exactly. This ensures the elimination of redundant interfering data and maximally enhances the accuracy of the results.
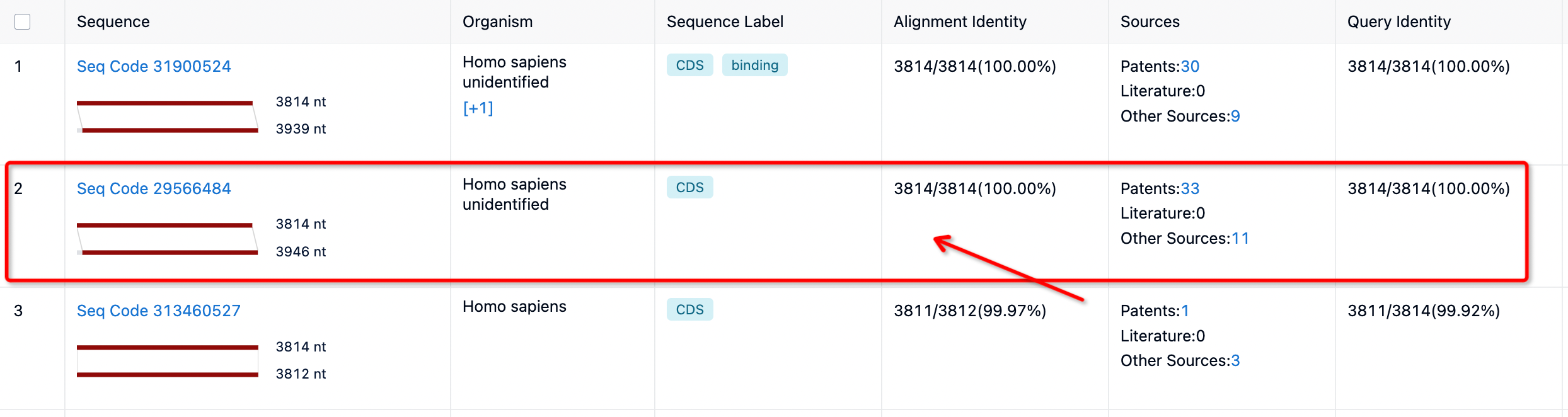
You can select the species source you wish to retrieve based on your needs. For instance, if we want to view the sequence information from humans, we select Homo sapiens. If the retrieved information needs to match the target sequence exactly, select 100% match. These functions can be performed through sequence filtering and screening on the left toolbar.
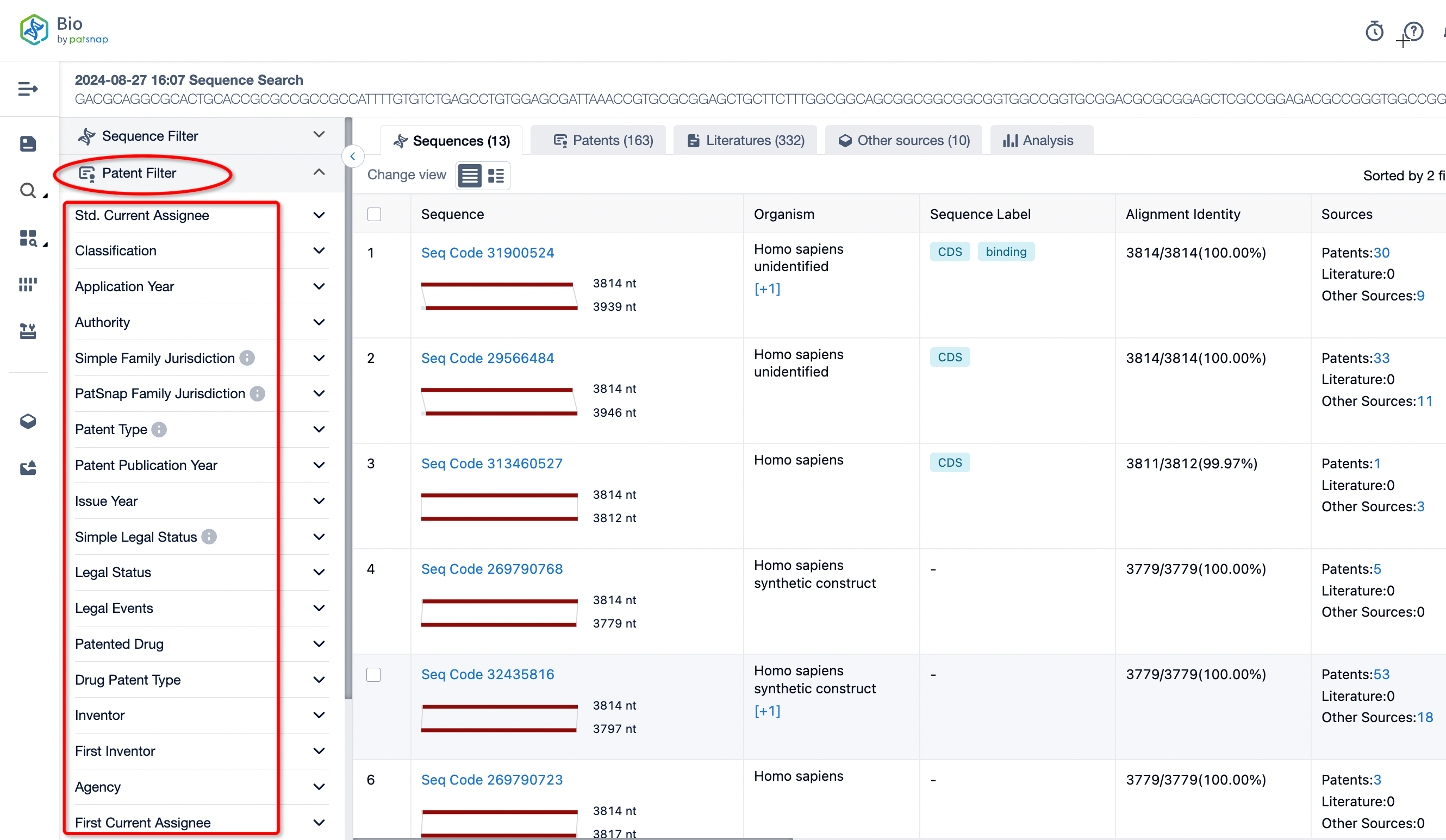
If you are interested in patents related to the sequence, you can directly click on the patent filter on the left side to filter detailed patent information according to your needs.
If you wish to look up literature related to a particular sequence or are interested in research information about the sequence, you can directly click on "View Literature" on the left. There, literature is categorized according to different databases. You can also filter by different authors, journals, institutions, and publication years, among other criteria, to quickly understand the research and development status of the relevant sequence.
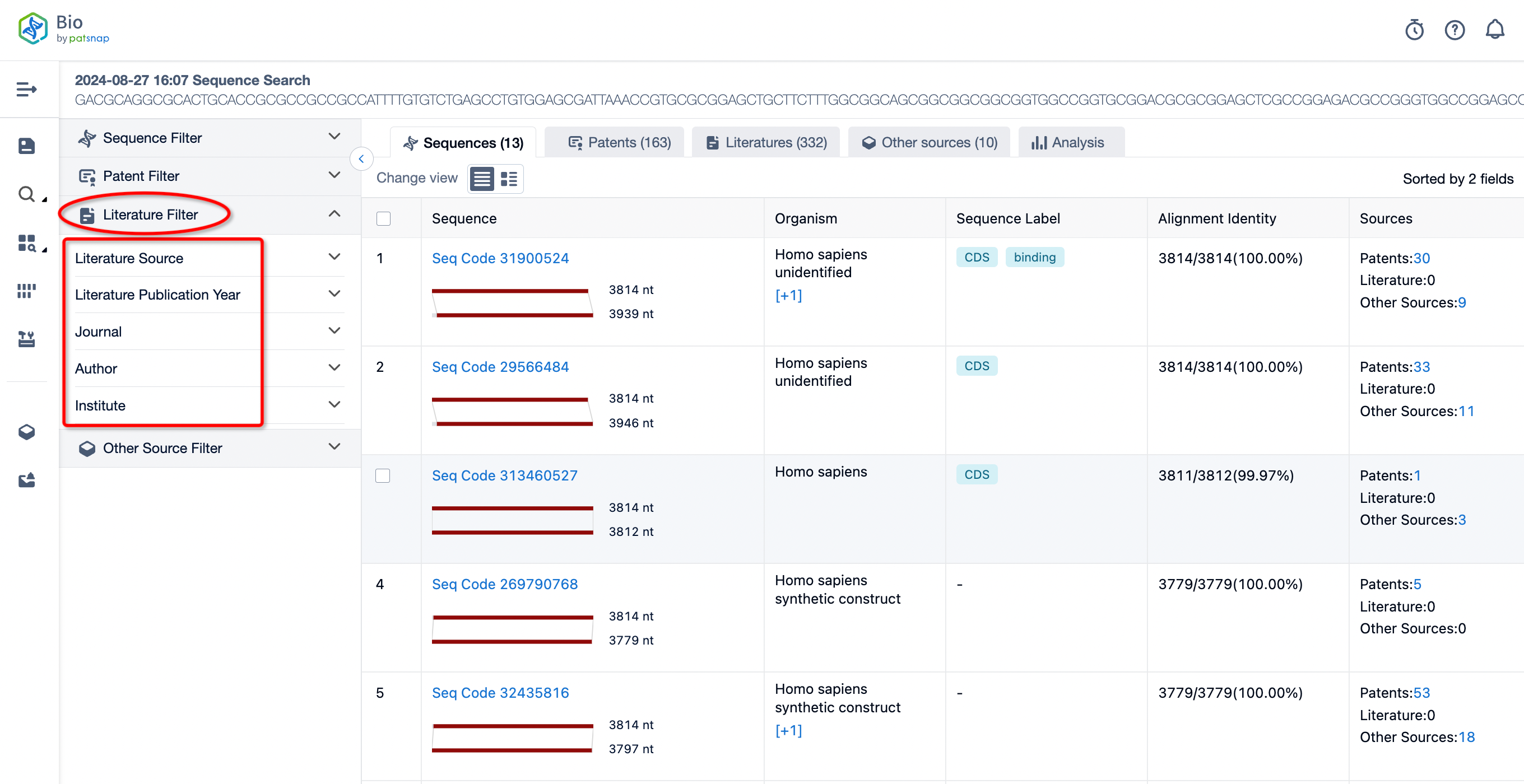
Additionally, Patsnap Bio also supports linking to other databases, enabling comprehensive analysis by interconnecting with databases like GENBANK, CAS, and EBI for a more detailed examination of the descriptions and records of related sequences held within these databases. It allows users to conduct cross-database linkage analysis to gain a complete understanding of nucleotide sequences, easily helping you resolve sequence retrieval challenges.
Summary
mRNA therapies are leading medical innovations, offering unprecedented speed and precision in providing new methods for the treatment and prevention of diseases, demonstrating their potential application across a wide range of disease prevention and treatment areas. The use of Patsnap Bio has expedited the retrieval of nucleotide sequences and the in-depth analysis of patent information, providing robust support for researchers and advancing the development of mRNA pharmaceuticals. This contributes to the infinite possibilities for the future of the healthcare sector.
Better answers for better bio-innovations!
Validate novelty, eliminate risk, and innovate with confidence using the world’s largest sequence database curated from millions of patent and non-patent sources.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.
With best-in-class coverage of protein and nucleic acid sequences combined with state-of- the-art search algorithms, you’ll spend less time searching and more time bringing your bio-innovations to market.